What the FD&C Act Means for Personal Care and OTC Product Companies

Overview
The FD&C Act exerts a profound influence on personal care and OTC product companies by mandating safety and effectiveness standards that must be satisfied prior to market entry. This article delineates how the FDA’s regulatory authority under this Act guarantees adherence through stringent testing, labeling requirements, and continuous surveillance. Such measures not only safeguard public health but also uphold the integrity of the industry, underscoring the critical nature of compliance in today’s market landscape.
Introduction
The Federal Food, Drug, and Cosmetic Act (FD&C Act) has been a pivotal element in the landscape of U.S. public health since its inception in 1938, fundamentally shaping the regulatory framework for personal care and over-the-counter (OTC) products. This legislation not only mandates rigorous safety and efficacy standards but also places the onus on manufacturers to navigate a complex web of compliance requirements.
As the industry evolves, challenges arise—how can companies balance innovation with stringent regulations while ensuring consumer trust and safety?
Exploring the implications of the FD&C Act reveals critical insights into the future of personal care and OTC product companies in an increasingly scrutinized market.
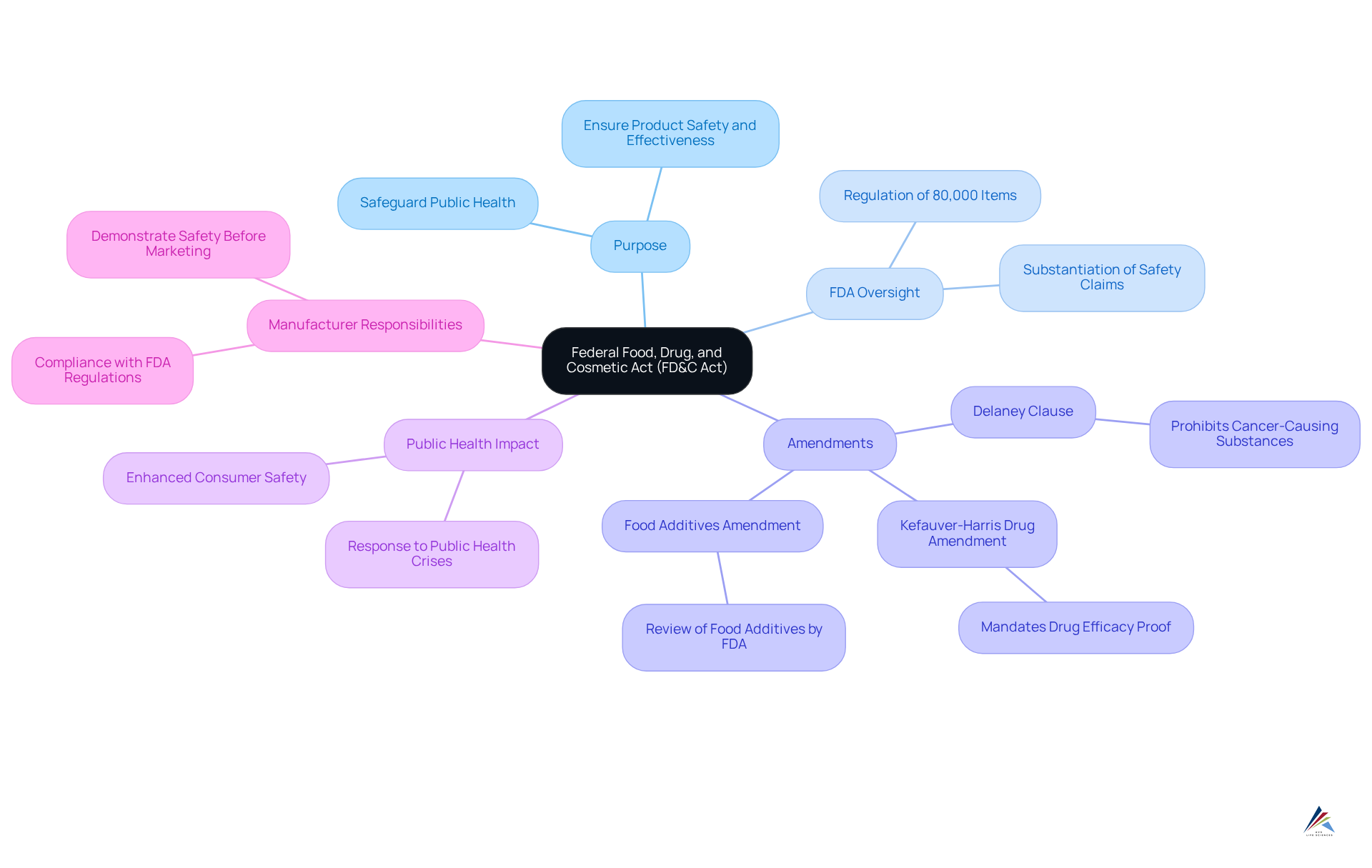
Define the FD&C Act and Its Purpose
Established in 1938, the Federal Food, Drug, and Cosmetic Act (FD&C Act) serves as the cornerstone of U.S. legislation that regulates the quality and effectiveness of food, drugs, and cosmetics, highlighting what the FD&C Act means for personal care and OTC product companies. Its primary objective is to safeguard public health by ensuring that what the FD&C Act means for personal care and OTC product companies is reflected in the safety and effectiveness of these products for consumption and their intended purposes.
What the FD&C Act means for personal care and OTC product companies is that the Act (FDA) to oversee these products, mandating that manufacturers substantiate safety and effectiveness claims prior to market entry. As of 2025, the FDA regulates approximately 80,000 items under the FD&C Act, underscoring its extensive reach.
The Act has undergone multiple amendments to address emerging public health challenges, highlighting what the FD&C Act means for personal care and OTC product companies, including the recent enhancements in 2025 that further bolster regulatory oversight. Notably, the Delaney Clause prohibits the inclusion of cancer-causing substances in food additives, while the Kefauver-Harris Drug Amendment compels drug manufacturers to demonstrate the efficacy of their products through rigorous research studies.
The FDA continues to enforce stringent regulations under the FD&C Act, which illustrates what the FD&C Act means for personal care and OTC product companies by ensuring a diverse array of items meets established safety standards and reinforcing its vital role in protecting public health. As FDA Commissioner Robert Califf articulated, 'The FD&C Act is essential in our mission to ensure that the items we consume are safe and effective, reflecting our commitment to public health.
Contextualize the FD&C Act in Regulatory Framework
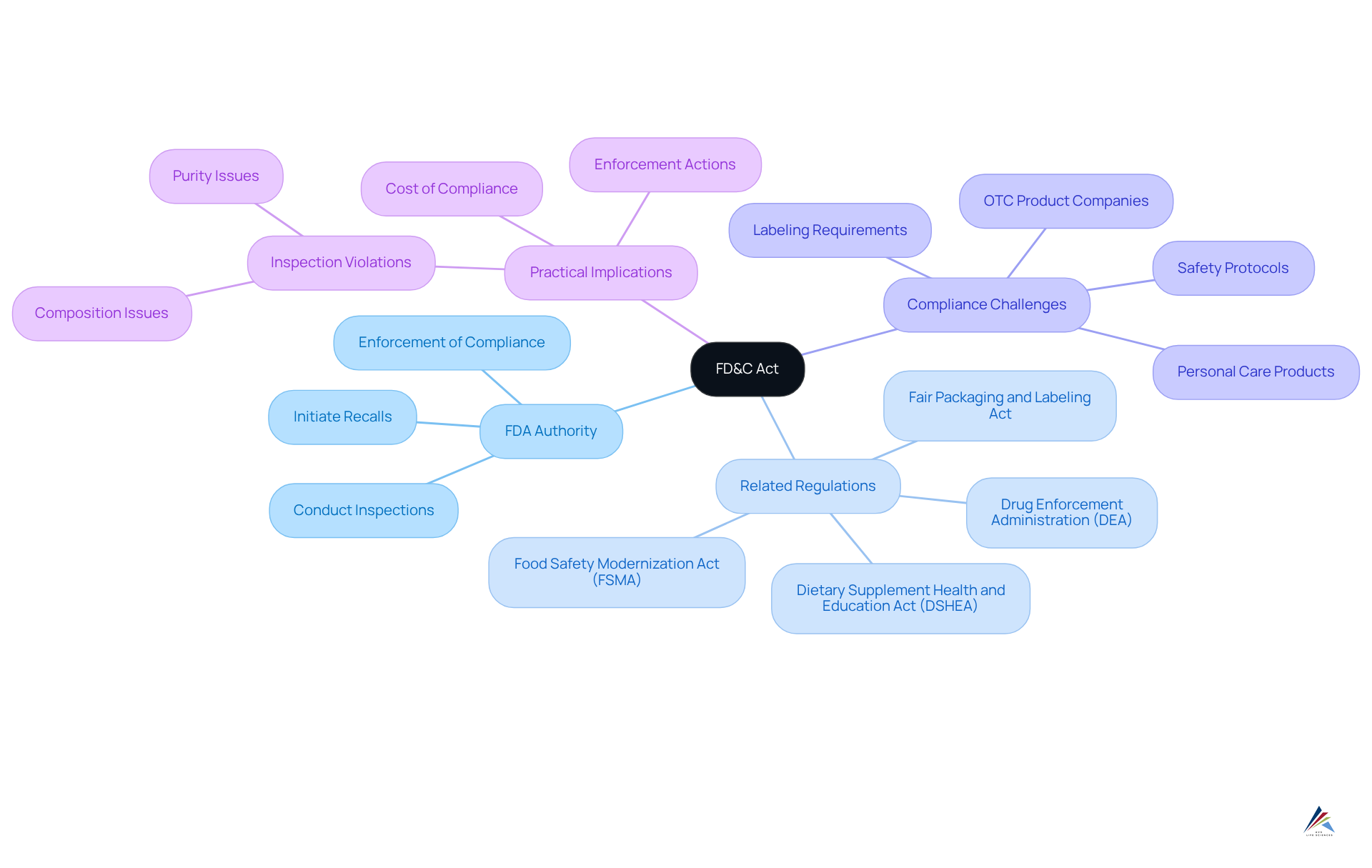
The FD&C Act serves as the cornerstone of the U.S. oversight system for health-related items, establishing the FDA's authority to enforce compliance, conduct inspections, and initiate recalls as necessary. This foundational legislation operates in conjunction with other essential regulations, such as those from the Drug Enforcement Administration (DEA) concerning controlled substances and the Fair Packaging and Labeling Act. Not only does the FD&C Act lay the groundwork for the FDA's oversight capabilities, but it also influences subsequent laws, including the Dietary Supplement Health and Education Act (DSHEA) and the Food Safety Modernization Act (FSMA).
These regulations delineate the regulatory framework for specific categories, ensuring that health items adhere to stringent standards for safety and labeling. Companies frequently encounter compliance challenges under the FD&C Act, which includes understanding what the FD&C Act means for personal care and OTC product companies, particularly in navigating the complexities of labeling requirements and maintaining adherence to safety protocols.
Failure to manage these aspects can lead to enforcement actions. For instance, in 2017, the FDA inspected 656 dietary supplement manufacturing sites, uncovering violations in over half of them, primarily related to purity and composition. This highlights what the FD&C Act means for personal care and OTC product companies in terms of .
Trace the Historical Development of the FD&C Act
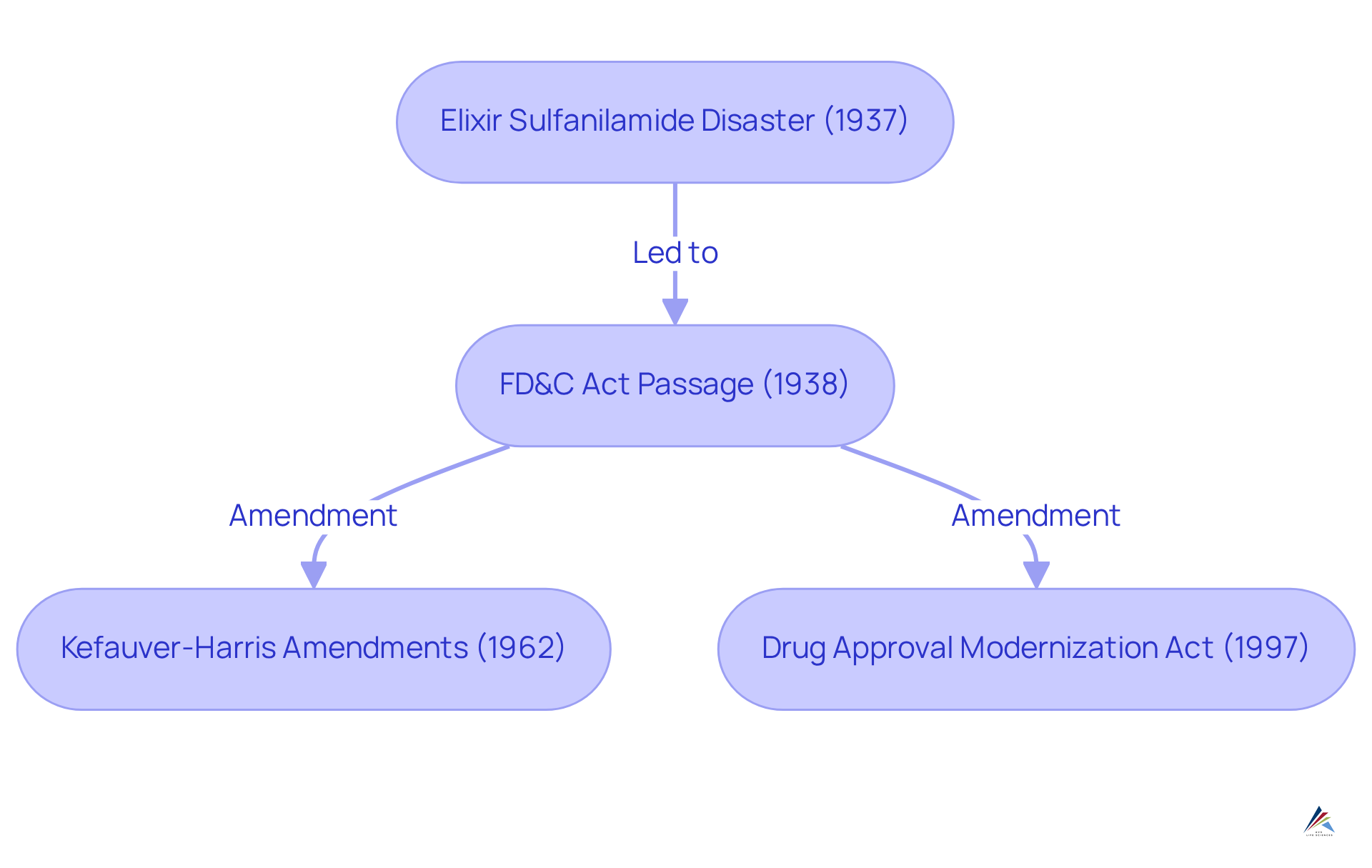
The FD&C Act arose from significant public concern over unsafe products, particularly following the 1937 Elixir Sulfanilamide disaster, which resulted in over 100 deaths due to a toxic formulation. This tragic event compelled Congress to act, culminating in the passage of the Act in 1938. Throughout the years, the FD&C Act has undergone numerous amendments to address , including the emergence of new drug classes, the proliferation of dietary supplements, and the demand for stricter safety standards. Noteworthy changes include:
- The Kefauver-Harris Amendments of 1962, which required manufacturers to prove the effectiveness of their products.
- The Drug Approval Modernization Act of 1997, which streamlined the approval process for new medications.
These modifications underscore the ongoing commitment to ensuring public safety and compliance in the pharmaceutical landscape.
Identify Key Components and Requirements of the FD&C Act
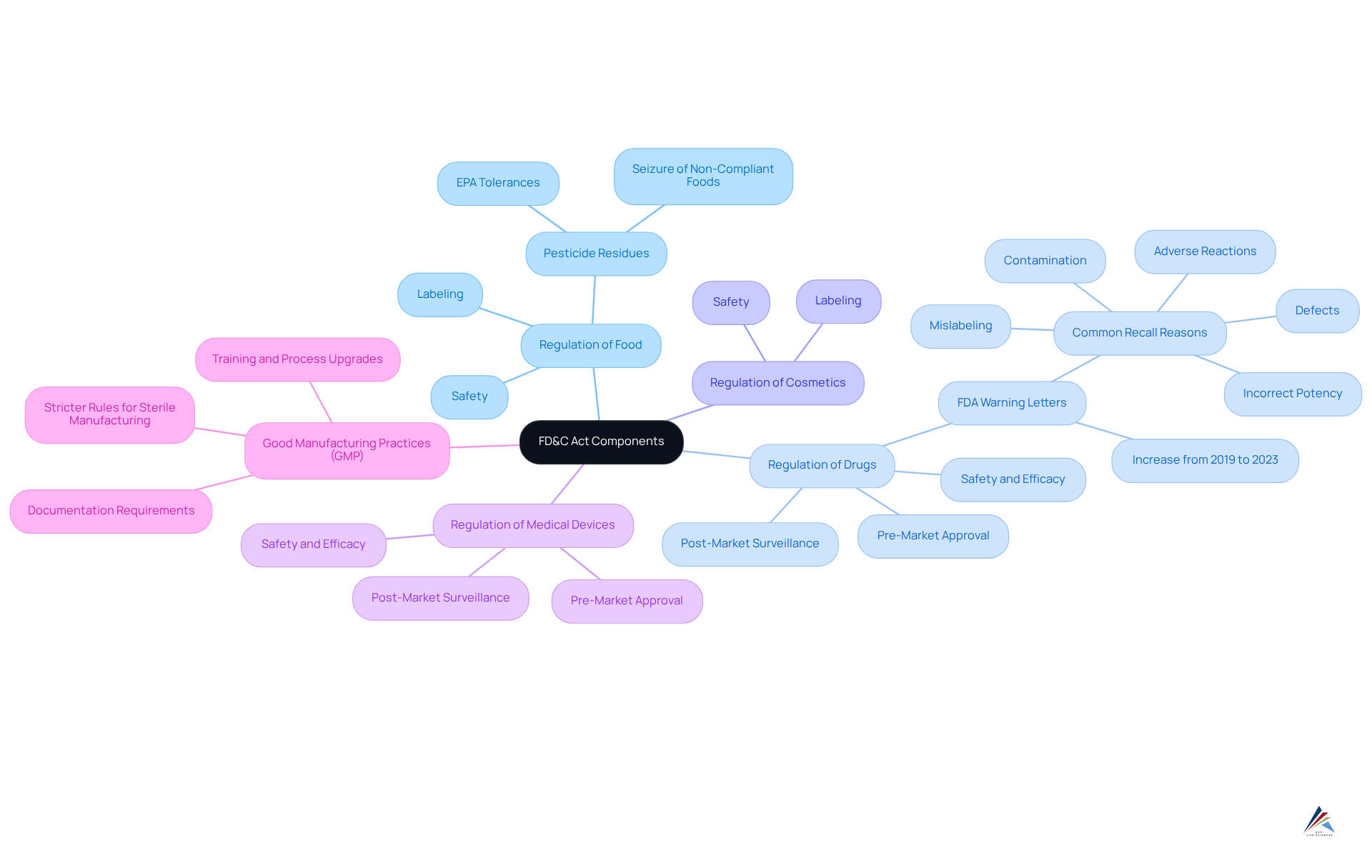
Key components of the FD&C Act encompass the regulation of food, drugs, cosmetics, and medical devices. This Act mandates that all items must be safe for use, accurately labeled, and produced in compliance with Good Manufacturing Practices (GMP). Furthermore, it necessitates pre-market approval for new drugs and medical devices, which involves rigorous testing and documentation to demonstrate safety and efficacy. Companies are also required to adhere to post-market surveillance requirements, ensuring ongoing compliance and reporting any adverse events associated with their products.
As we approach 2025, updates to GMP requirements highlight the need for enhanced documentation, rigorous testing protocols, and the imperative for companies to invest in training and process upgrades to meet evolving standards. The EU GMP guidelines Volume 4 introduces stricter rules for sterile manufacturing and environmental monitoring, underscoring the critical importance of compliance in today’s oversight landscape. Moreover, from 2019 to 2023, the FDA has increased the issuance of warning letters from 2.98 to 4.27 per 100 inspections, signifying an intensification of oversight.
This rigorous framework not only but also reinforces the integrity of the pharmaceutical and personal care industries. Engaging with compliance solutions is not merely advisable; it is essential for maintaining industry standards and ensuring the safety and efficacy of products in the market.
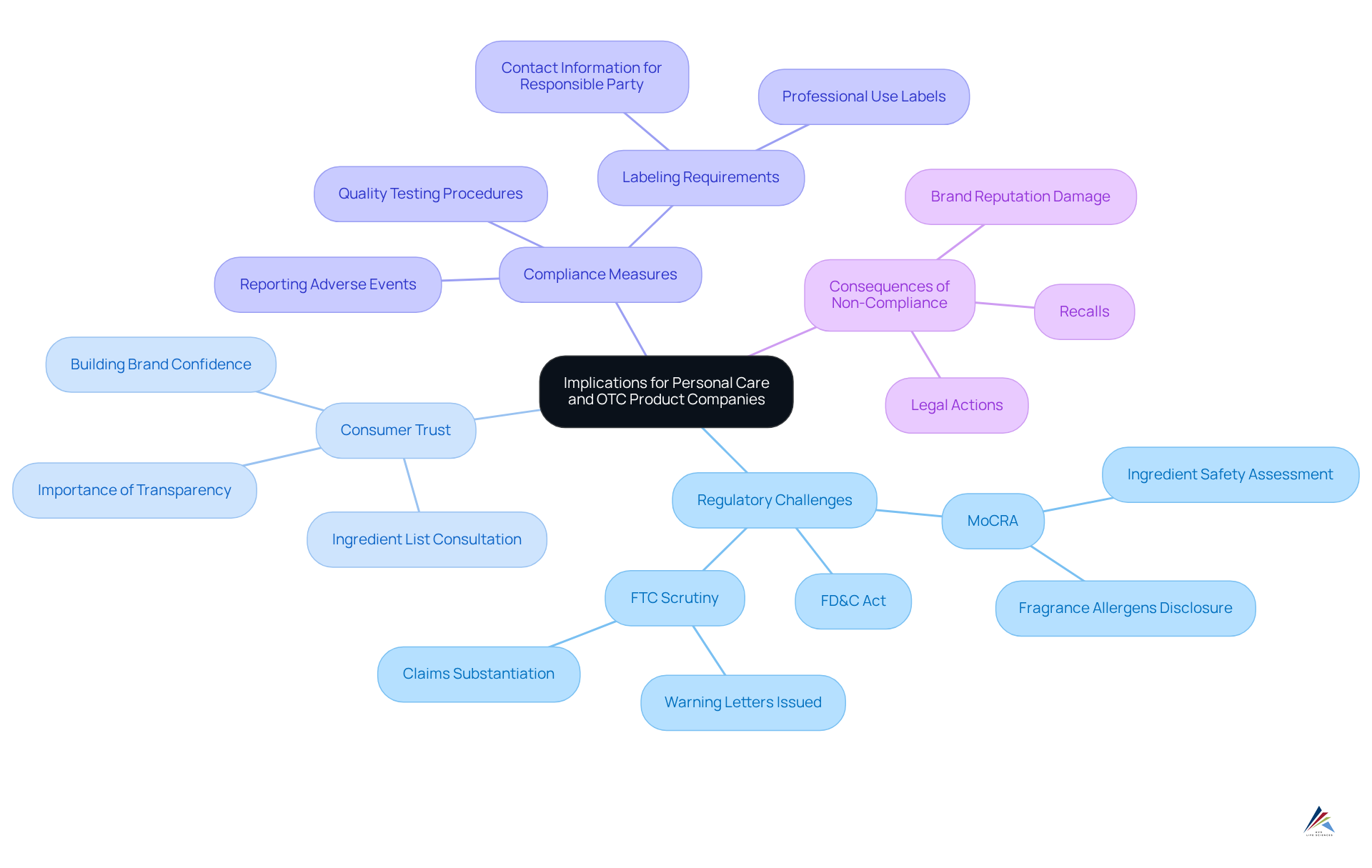
Discuss Implications for Personal Care and OTC Product Companies
Understanding what the FD&C Act means for personal care and OTC product companies is paramount for ensuring market access and safeguarding consumer health. These firms face numerous regulatory challenges, including stringent standards for formulation, labeling, and marketing claims. The repercussions of non-compliance can be severe, resulting in recalls, legal actions, and significant damage to brand reputation. Notably, the U.S. Federal Trade Commission (FTC) has issued warning letters to over 700 beauty and personal care brands regarding dubious marketing claims, highlighting the increased scrutiny within the industry.
Furthermore, staying informed about evolving regulations and industry standards is essential for maintaining compliance. The FD&C Act not only governs product effectiveness and safety but also illustrates what the FD&C Act means for personal care and OTC product companies in terms of shaping innovation within the sector. Companies must adeptly navigate the fine line between launching new products and adhering to rigorous safety standards. As emphasized by industry experts, verifying claims about products is not merely a compliance obligation; it is also a vital strategy for building consumer trust and confidence in the brand.
With the introduction of (MoCRA), companies are urged to prioritize transparency and accountability in their operations to meet both regulatory and consumer expectations. Additionally, it is noteworthy that over 70% of shoppers consult the ingredient list before making a purchase, underscoring the critical importance of transparency in compliance. Companies must also recognize the new requirement to report serious adverse events within 15 business days, further highlighting the necessity for robust compliance measures.
Conclusion
The Federal Food, Drug, and Cosmetic Act (FD&C Act) serves as a vital regulatory framework that ensures the safety and efficacy of personal care and over-the-counter (OTC) products. Its comprehensive guidelines empower the FDA to enforce standards that protect public health, illustrating the importance of compliance for companies in this sector. By mandating rigorous testing and accurate labeling, the FD&C Act not only safeguards consumers but also shapes the landscape of the personal care industry.
Throughout this article, key aspects of the FD&C Act have been explored, including its historical context, regulatory implications, and the evolving requirements that companies must navigate. The Act's amendments reflect a continuous commitment to addressing emerging health challenges, while its enforcement mechanisms underscore the seriousness of compliance. Companies face significant challenges in adhering to these regulations, from formulation and labeling to marketing claims, with non-compliance potentially leading to severe repercussions.
Ultimately, the FD&C Act is more than just a set of regulations; it represents a commitment to public health and consumer safety. As personal care and OTC product companies adapt to the evolving regulatory landscape, they must prioritize transparency and accountability. By doing so, they not only ensure compliance but also foster consumer trust, which is essential for success in a competitive marketplace. Embracing these principles will be crucial as the industry moves forward, particularly in light of the upcoming changes in 2025.
Frequently Asked Questions
What is the Federal Food, Drug, and Cosmetic Act (FD&C Act)?
The FD&C Act, established in 1938, is a key piece of U.S. legislation that regulates the quality and effectiveness of food, drugs, and cosmetics, aiming to safeguard public health.
What is the primary purpose of the FD&C Act?
The primary purpose of the FD&C Act is to ensure the safety and effectiveness of food, drugs, and cosmetics for consumption and their intended uses.
What role does the FDA play under the FD&C Act?
The FDA is empowered by the FD&C Act to oversee food, drug, and cosmetic products, requiring manufacturers to substantiate safety and effectiveness claims before these products can enter the market.
How many items does the FDA regulate under the FD&C Act as of 2025?
As of 2025, the FDA regulates approximately 80,000 items under the FD&C Act.
What are some key amendments to the FD&C Act?
Key amendments include the Delaney Clause, which prohibits cancer-causing substances in food additives, and the Kefauver-Harris Drug Amendment, which requires drug manufacturers to demonstrate product efficacy through rigorous research studies.
How does the FD&C Act fit into the broader regulatory framework?
The FD&C Act is foundational to the U.S. oversight system for health-related items, establishing the FDA's authority to enforce compliance, conduct inspections, and initiate recalls, while influencing other regulations like the DSHEA and FSMA.
What challenges do companies face under the FD&C Act?
Companies often face compliance challenges related to understanding labeling requirements and adhering to safety protocols, which are crucial for meeting the standards set by the FD&C Act.
What are the consequences of failing to comply with the FD&C Act?
Failure to manage compliance with the FD&C Act can lead to enforcement actions, as evidenced by the FDA's inspection of dietary supplement manufacturing sites in 2017, where over half were found to have violations related to purity and composition.
