What is CMC in Pharma: Key Concepts and Importance Explained

Overview
The article elucidates that CMC, or Chemistry, Manufacturing, and Controls, is indispensable in the pharmaceutical sector, as it encompasses the essential processes and documentation required to ensure the safety, efficacy, and quality of drug products. A thorough understanding and adherence to CMC regulations are crucial for compliance with industry standards and successful regulatory submissions, thereby safeguarding patient health and facilitating efficient drug development. This highlights the pressing compliance challenges faced by professionals in the field and underscores the need for robust solutions to navigate them effectively.
Introduction
Understanding the intricate web of Chemistry, Manufacturing, and Controls (CMC) is essential for anyone navigating the pharmaceutical landscape. As the industry faces heightened regulatory scrutiny and complex drug development processes, grasping the nuances of CMC becomes increasingly critical. What happens when this fundamental framework is overlooked? The stakes are high; potential delays in drug approvals and significant impacts on patient safety underscore the imperative to explore the pivotal role CMC plays in ensuring the efficacy and quality of pharmaceutical products.
Define CMC: Core Concepts and Significance in Pharmaceuticals
What is CMC in pharma? It encompasses a broad spectrum of activities and procedures vital to the development and production of pharmaceutical products. This includes the chemical composition of substances, the manufacturing processes employed, and the controls established to ensure quality and consistency throughout production. Understanding what is CMC in pharma is paramount; it is crucial for , thereby protecting patient health and ensuring adherence to regulatory mandates.
As we approach 2025, knowing what is CMC in pharma is indispensable for medication development, particularly as the industry faces increasing scrutiny from regulatory bodies. Inadequate or incomplete CMC documentation can result in significant delays in approval timelines or even outright rejections. For example, the CMC section of a New Drug Application (NDA) must strictly comply with FDA regulatory requirements, as demonstrated by the challenges faced by Ultragenyx Pharmaceutical Inc. with its UX111 gene therapy, which received a Complete Response Letter (CRL) due to specific CMC observations.
Understanding what is CMC in pharma highlights the necessity of tailored methods for each candidate, as a one-size-fits-all strategy is impractical. Collaborating with contract manufacturing organizations is often essential for innovative companies that lack extensive in-house CMC resources. Moreover, meticulous documentation of all CMC activities, including adherence to Great Documentation Practices and Standard Operating Procedures (SOPs), is critical for successful product development and regulatory submissions, ensuring consistency and reliability in the development process.
Statistics underscore the intricate nature of drug development, revealing that only one or two out of every 10,000 compounds analyzed ultimately receive approval. This underscores the vital role that CMC plays in navigating the complexities of product development and regulatory approval, which raises the question of what is CMC in pharma, solidifying its status as a cornerstone of the industry. AVS Life Sciences, with its expertise in GMP audits and GXP regulatory services, guarantees zero findings during audits, highlighting what is CMC in pharma as essential for maintaining quality and compliance.
Trace the Evolution of CMC: Historical Context and Development
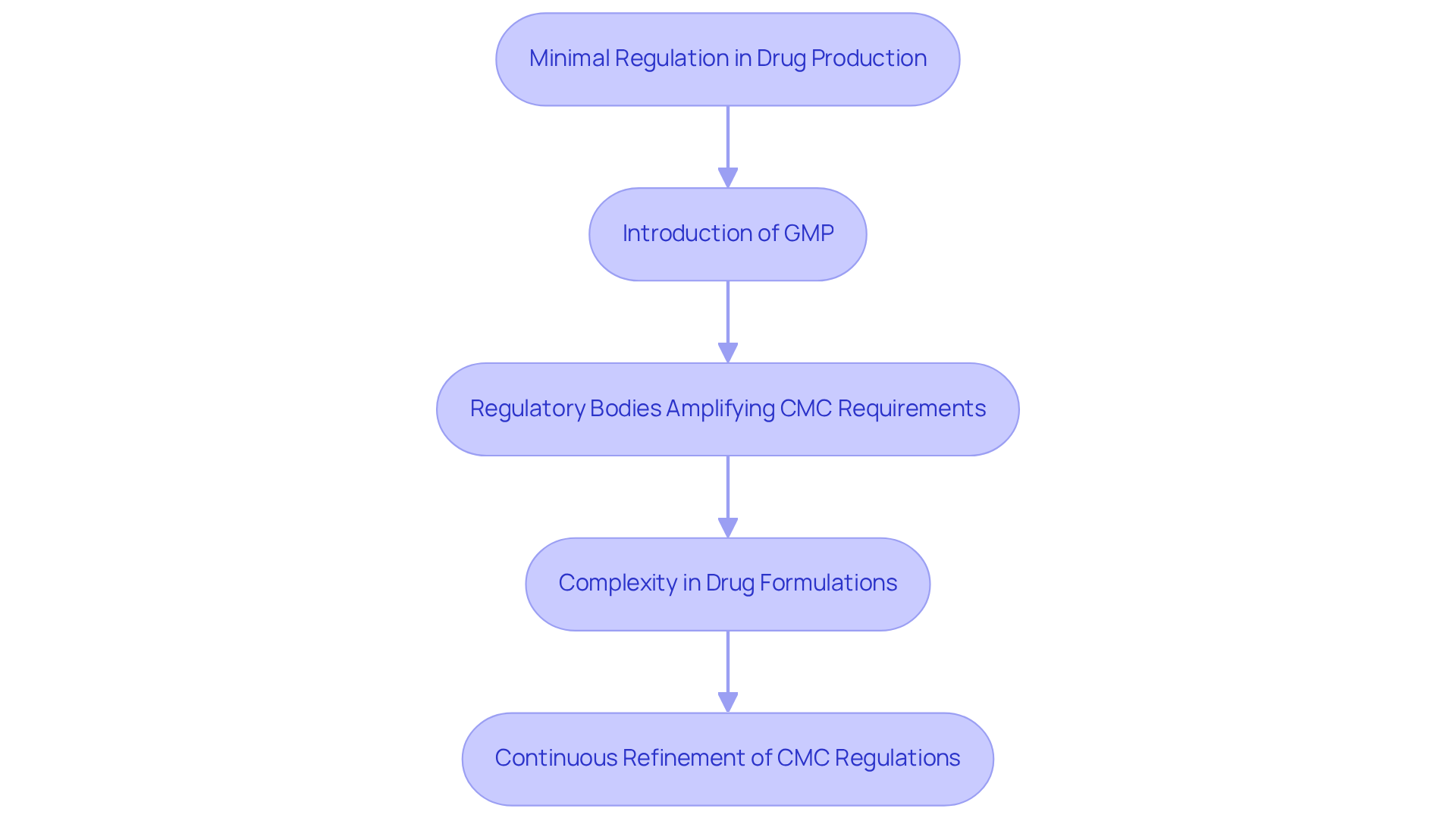
Understanding what is cmc in pharma has been pivotal in the drug industry, particularly since the mid-20th century when Good Manufacturing Practices (GMP) were instituted. Initially, drug production functioned with minimal regulation, leading to significant inconsistencies in product quality and safety. The establishment of GMP marked a transformative shift, introducing stringent guidelines that manufacturers must adhere to in order to ensure the integrity of pharmaceutical products. Regulatory bodies, such as the FDA and EMA, have since amplified CMC requirements, emphasizing what is cmc in pharma as essential for thorough documentation and robust quality assurance measures.
This evolution transcends mere procedure; it embodies a growing acknowledgment of and its vital role in ensuring safety and efficacy. As drug formulations and manufacturing techniques have become increasingly complex, understanding what is cmc in pharma has gained more emphasis. For example, AVS Life Sciences' successful upgrade of a biotechnology GMP facility exemplifies how the implementation of GMP has resulted in enhanced product consistency and compliance, ultimately bolstering patient safety. AVS Life Sciences collaborated with a leading biotechnology company to elevate their manufacturing environment from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. During this process, challenges such as the misalignment of barcode scanner cameras were identified, initially causing inconsistent test results. This oversight underscored the importance of rigorous testing and quality checks. The project was completed on time and within budget while ensuring full traceability through meticulous documentation, and the lessons learned prompted the QC laboratory team to further evaluate their business processes.
Furthermore, understanding what is cmc in pharma is essential as the continuous refinement of CMC regulations adapts to the ever-evolving landscape of medicine manufacturing, ensuring that quality remains paramount in product development. As highlighted by industry specialists, what is cmc in pharma plays an essential role in every phase of medication development, assisting producers in creating safe and effective medical products. The increasing complexity of pharmaceutical production accentuates the growing significance of understanding what is CMC in pharma, as it provides a scientific basis for regulating manufacturing processes and ensuring product safety and efficacy.
Examine Key Components of CMC: Processes and Regulatory Framework
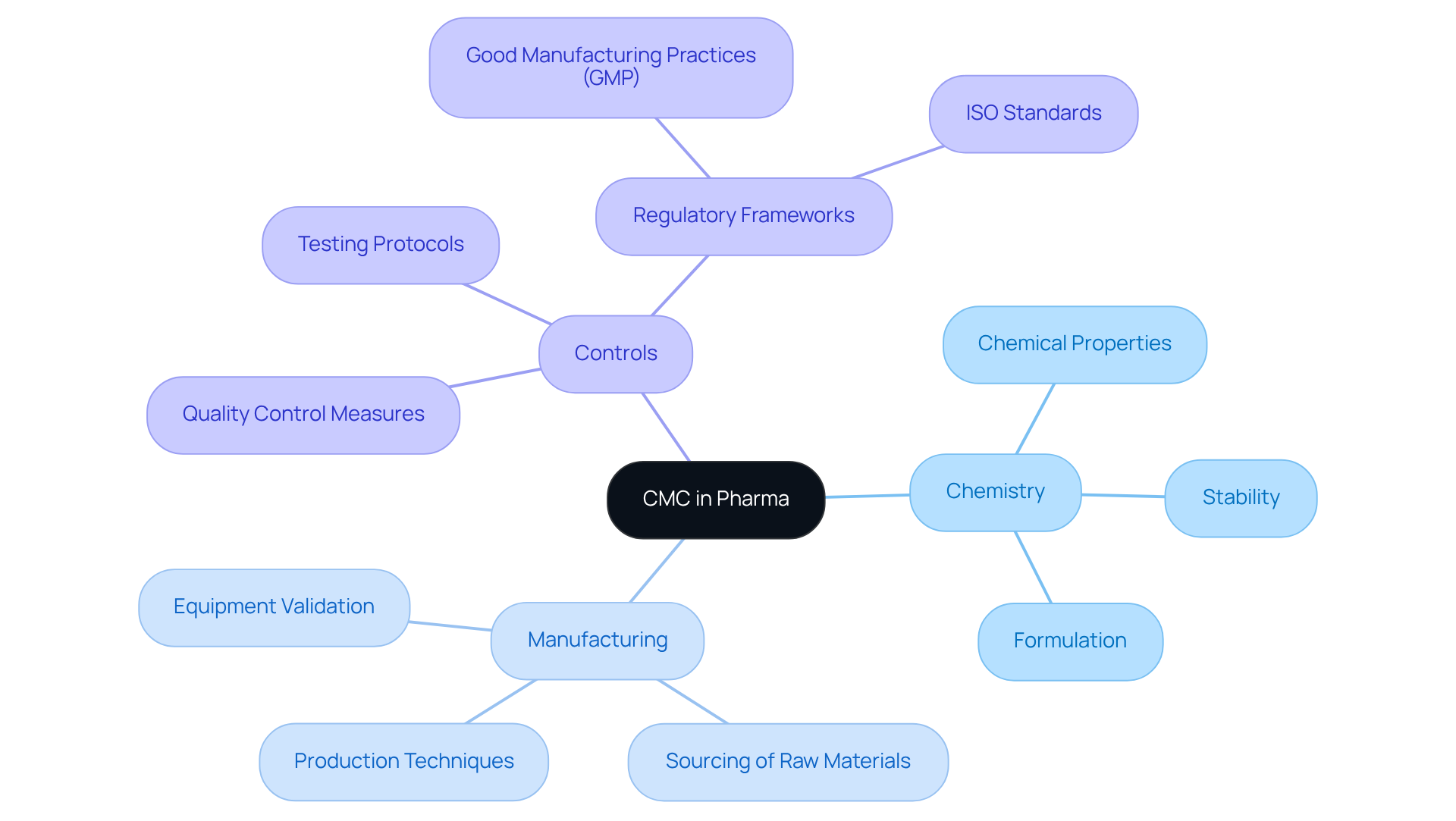
Understanding what is CMC in pharma is critical to grasping the key components that influence compliance in pharmaceutical development:
- Chemistry: This encompasses the comprehensive characterization of the pharmaceutical substance, emphasizing its chemical properties, stability, and formulation. A thorough understanding of these aspects is crucial for ensuring that the medication functions as intended, safeguarding both efficacy and safety throughout its lifecycle.
- Manufacturing: This involves the various methods employed to create the drug, including the sourcing of raw materials, production techniques, and equipment validation. Consistency in manufacturing practices is vital to meet stringent regulatory standards, ensuring that each batch adheres to predefined quality specifications.
- Controls: This pertains to the quality control measures implemented throughout the manufacturing process, which include rigorous testing and validation protocols. Regulatory frameworks, such as [Good Manufacturing Practices (GMP)](https://avslifesciences.com/industry-news/automation-validation-solutions-llc-acquires-mwa-consulting-inc) and ISO standards, guide these controls to guarantee that every batch of medication is safe and effective.
Together, these components form the backbone of CMC, which relates to what is cmc in pharma, ensuring that medical products are developed and produced to the highest standards, ultimately contributing to public health and safety.
Analyze the Impact of CMC on Drug Development and Lifecycle Management
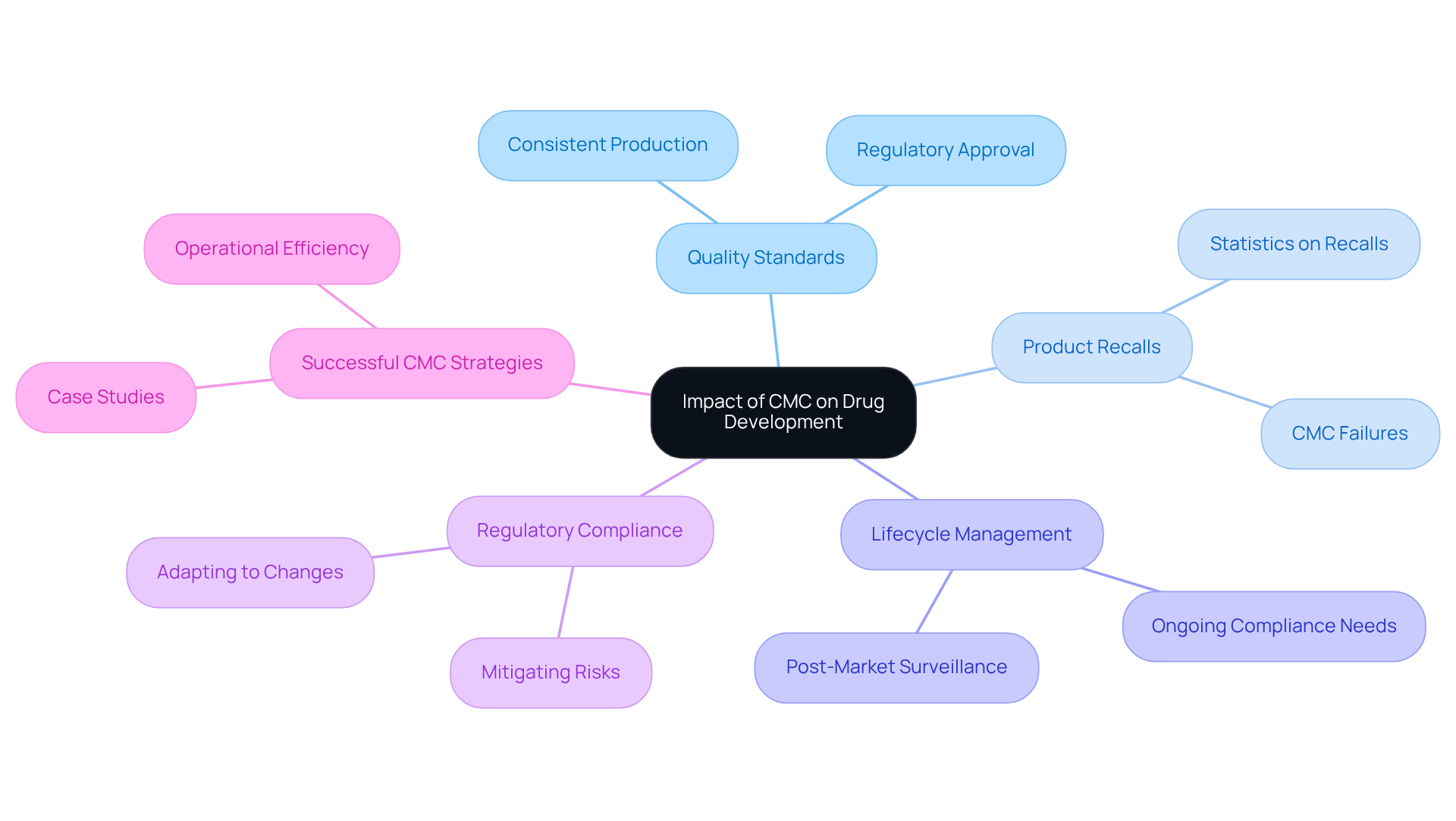
Understanding what is CMC in pharma reveals its profound and lifecycle management. Effective CMC practices are crucial for understanding what is CMC in pharma, as they ensure that medications are consistently produced to meet rigorous quality standards, which is critical for regulatory approval.
Alarmingly, statistics reveal that a significant percentage of product recalls are tied to CMC failures, which raises the question of what is CMC in pharma and emphasizes the necessity of robust CMC strategies in mitigating risks associated with regulatory penalties and market delays.
Furthermore, understanding what is CMC in pharma plays a vital role in lifecycle management; ongoing compliance with CMC requirements is crucial for post-market surveillance and necessary product modifications.
Understanding what is CMC in pharma allows companies to not only bolster their ability to adapt to regulatory changes and market demands but also enhance patient outcomes and drive business success.
Recent case studies illustrate what is CMC in pharma, demonstrating that organizations implementing successful CMC strategies have experienced fewer recalls and greater operational efficiency, thereby reinforcing the value of a strong CMC framework in the pharmaceutical industry.
Conclusion
Understanding CMC in pharmaceuticals is essential for ensuring the safety, efficacy, and regulatory compliance of drug products. This discussion emphasizes that CMC encompasses a wide range of activities critical to the development and production of pharmaceuticals, highlighting its role in maintaining quality and consistency throughout the manufacturing process. As the pharmaceutical landscape evolves, the importance of CMC becomes increasingly pronounced, serving as a fundamental pillar for successful drug development.
Key insights reveal that CMC is not merely about adhering to regulatory requirements; it involves a comprehensive understanding of chemistry, manufacturing processes, and quality controls. The historical context demonstrates how the evolution of CMC practices has shaped the industry, moving from minimal regulation to stringent guidelines that prioritize patient safety. Furthermore, the impact of CMC on drug development and lifecycle management underscores the necessity of robust strategies to mitigate risks associated with regulatory failures and product recalls.
In conclusion, the significance of CMC in pharmaceuticals cannot be overstated. As the industry faces growing scrutiny and complexity, stakeholders must prioritize the implementation of effective CMC practices to enhance patient outcomes and ensure compliance with evolving regulations. A strong CMC framework is not only vital for securing regulatory approvals but also essential for fostering innovation and maintaining public trust in pharmaceutical products. Embracing these principles will ultimately lead to a more reliable and effective healthcare system.
Frequently Asked Questions
What does CMC stand for in pharmaceuticals?
CMC stands for Chemistry, Manufacturing, and Controls, which encompasses a broad spectrum of activities and procedures vital to the development and production of pharmaceutical products.
Why is CMC important in the pharmaceutical industry?
CMC is crucial for ensuring that each batch of medication meets rigorous safety and efficacy standards, thereby protecting patient health and ensuring adherence to regulatory mandates.
What are the key components of CMC?
Key components of CMC include the chemical composition of substances, the manufacturing processes employed, and the controls established to ensure quality and consistency throughout production.
What challenges can arise from inadequate CMC documentation?
Inadequate or incomplete CMC documentation can result in significant delays in approval timelines or even outright rejections of drug applications.
Can you provide an example of a company that faced CMC-related challenges?
Ultragenyx Pharmaceutical Inc. faced challenges with its UX111 gene therapy, receiving a Complete Response Letter (CRL) due to specific CMC observations.
Why is a tailored approach to CMC necessary?
A tailored approach is necessary because each candidate drug is unique, and a one-size-fits-all strategy is impractical in addressing the specific CMC needs of different pharmaceutical products.
How do contract manufacturing organizations (CMOs) fit into CMC?
Collaborating with contract manufacturing organizations is often essential for innovative companies that lack extensive in-house CMC resources.
What practices are critical for successful CMC documentation?
Meticulous documentation of all CMC activities, including adherence to Good Documentation Practices and Standard Operating Procedures (SOPs), is critical for successful product development and regulatory submissions.
What is the approval rate for compounds in drug development?
Statistics reveal that only one or two out of every 10,000 compounds analyzed ultimately receive approval, highlighting the complexity of drug development.
How does AVS Life Sciences contribute to CMC in pharma?
AVS Life Sciences provides expertise in GMP audits and GXP regulatory services, ensuring zero findings during audits, which emphasizes the importance of CMC for maintaining quality and compliance in the pharmaceutical industry.
