What is a Pharmaceutical Consultant? Key Roles and Skills Explained

Overview
Pharmaceutical consultants serve as specialized advisors, expertly guiding drug companies and healthcare organizations through the intricate processes of drug development, regulatory compliance, and quality assurance. Their essential roles are highlighted as they navigate complex regulations, enhance operational efficiency, and ensure product quality. This underscores their critical importance in the pharmaceutical industry, where compliance challenges are ever-present. By leveraging their expertise, these consultants not only address regulatory hurdles but also foster a culture of quality and efficiency, ultimately driving the success of pharmaceutical endeavors.
Introduction
Pharmaceutical consultants serve as the unsung heroes within the intricate drug development process, expertly guiding companies through the labyrinth of regulations and quality standards. As specialists in their field, they offer invaluable insights that not only streamline compliance but also enhance product quality and operational efficiency. Yet, a pivotal question arises: what specific roles do these consultants occupy in the dynamic pharmaceutical landscape, and what essential skills underpin their success in this critical profession?
Define Pharmaceutical Consultant: Key Roles and Functions
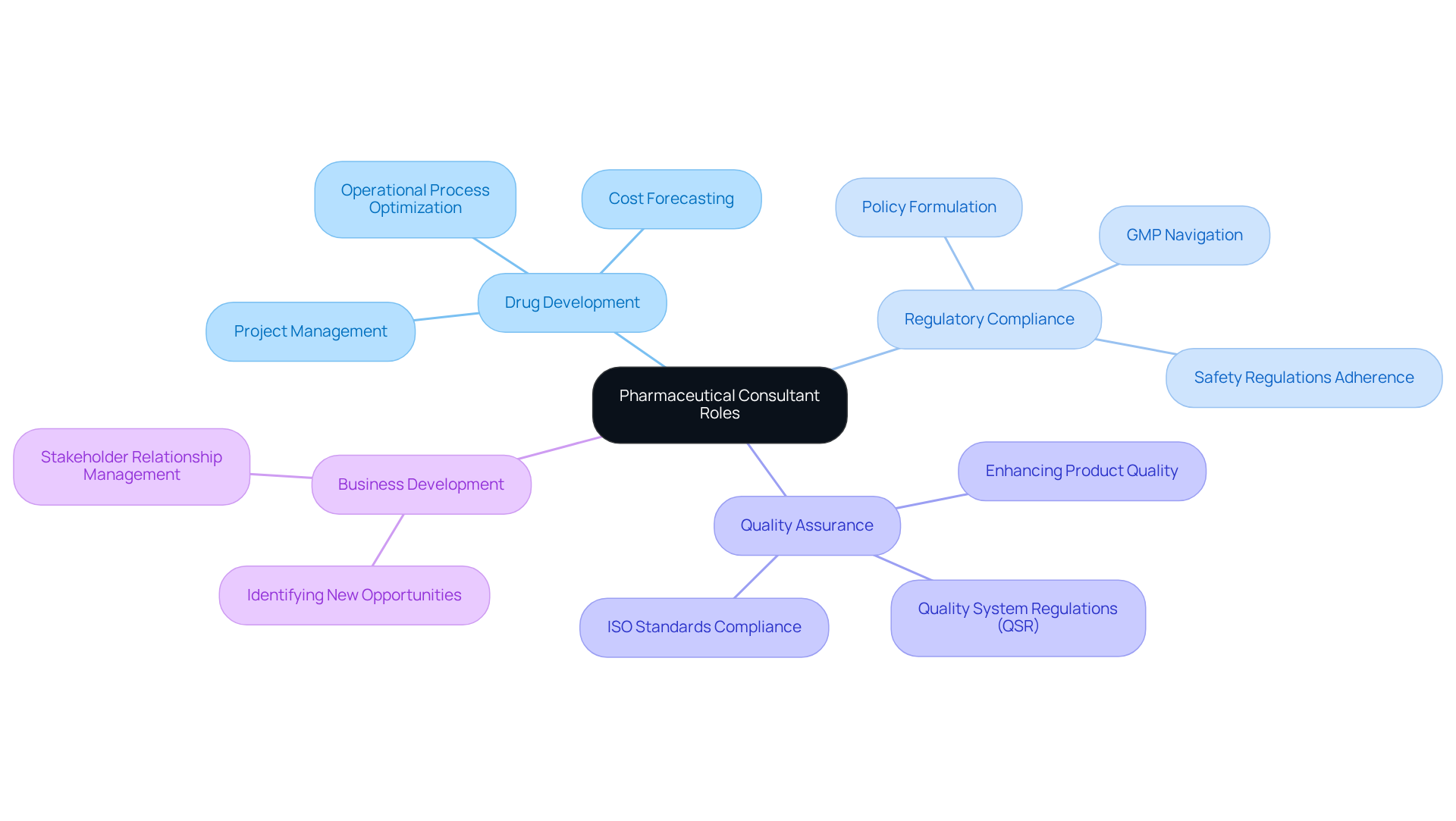
A pharmaceutical consultant serves as a specialized advisor, offering expert guidance to drug companies, healthcare organizations, and related agencies. Their primary responsibilities include steering clients through various facets of drug development, regulatory compliance, and quality assurance. This involves navigating intricate regulatory landscapes, ensuring adherence to (GMP), ISO standards, and Quality System Regulations (QSR), and optimizing operational processes. By leveraging their extensive industry expertise, pharmaceutical consultants significantly enhance product development strategies and refine regulatory frameworks.
For instance, advisors play a crucial role in elevating quality assurance throughout drug development. They support organizations in establishing robust operational processes that enhance efficiency and minimize waste, ultimately improving product quality. A noteworthy example is AVS Life Sciences's collaboration with a leading biotechnology company to upgrade their manufacturing facility from a Biosafety Level 1 GMP site to a Level 2 GMP facility. This project was completed on schedule and within budget, exemplifying AVS's commitment to quality assurance and adherence to industry standards.
Moreover, the importance of pharmaceutical consultants in ensuring regulatory compliance cannot be overstated. They assist in formulating company policies that guarantee adherence to safety regulations and government mandates, thereby preventing costly errors and safeguarding product safety. Industry leaders emphasize that the expertise of a pharmaceutical consultant is indispensable for navigating the complexities of product development. Their proficiency in forecasting costs linked to the development of new medications and medical devices enables companies to manage investments wisely and prepare for financial implications. As the pharmaceutical landscape continues to evolve, the role of pharmaceutical consultants, particularly those at AVS Life Sciences, in ensuring compliance and enhancing quality assurance remains essential for achieving success.
Contextualize the Role: Importance of Pharmaceutical Consultants in Compliance and Quality
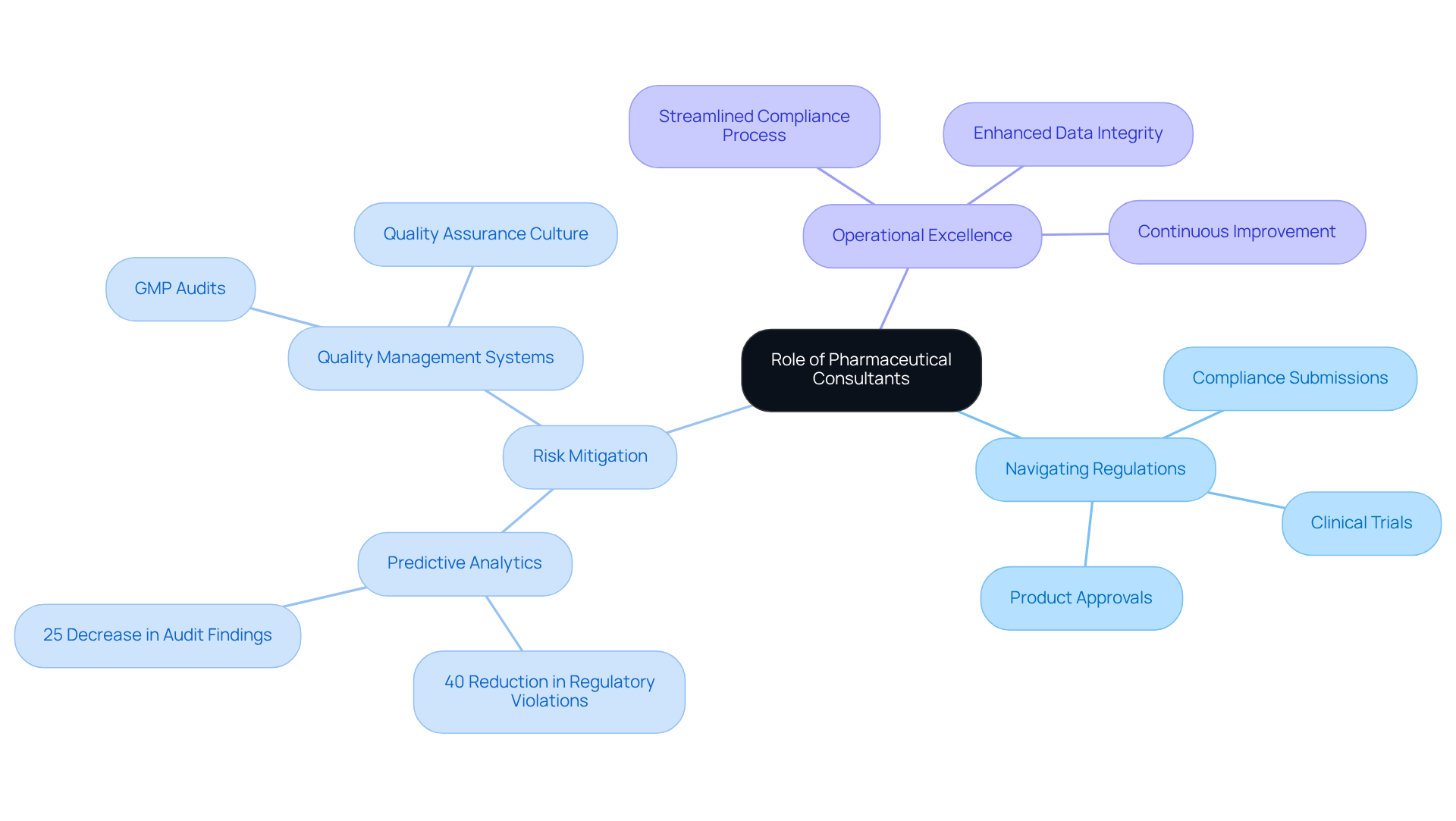
Pharmaceutical advisors play a pivotal role in helping firms navigate the stringent regulations imposed by organizations such as the FDA and EMA. Their specialized expertise is vital for guiding companies through the intricate processes of compliance submissions, clinical trials, and product approvals. By offering strategic insights and practical solutions, these advisors significantly mitigate the risks associated with non-compliance, which can result in costly delays and damage to a company's reputation. For instance, firms utilizing predictive analytics have reported a 40% reduction in regulatory violations and a 25% decrease in audit findings, underscoring the effectiveness of expert guidance in managing adherence to regulations.
The significance of pharmaceutical consultants is especially clear for startups and smaller enterprises that may not have the internal expertise needed to address these challenges. Their involvement not only streamlines the compliance process but also fosters a culture of quality assurance within organizations. AVS Life Sciences, as a dedicated partner in quality control, provides comprehensive GXP oversight services, including meticulous GMP audits that assess compliance in APIs, drug products, and testing facilities. These audits not only ensure adherence to established standards but also enhance data integrity and operational efficiency, ultimately benefiting clients by .
As noted by industry experts, the role of advisors extends beyond mere compliance; they are instrumental in elevating overall product quality and ensuring that organizations are adequately prepared for regulatory scrutiny. By implementing robust Quality Management Systems (QMS) and risk mitigation strategies, pharmaceutical consultants, including those at AVS Life Sciences, empower companies to achieve sustainable compliance and operational excellence.
Outline Responsibilities: Daily Tasks and Duties of Pharmaceutical Consultants
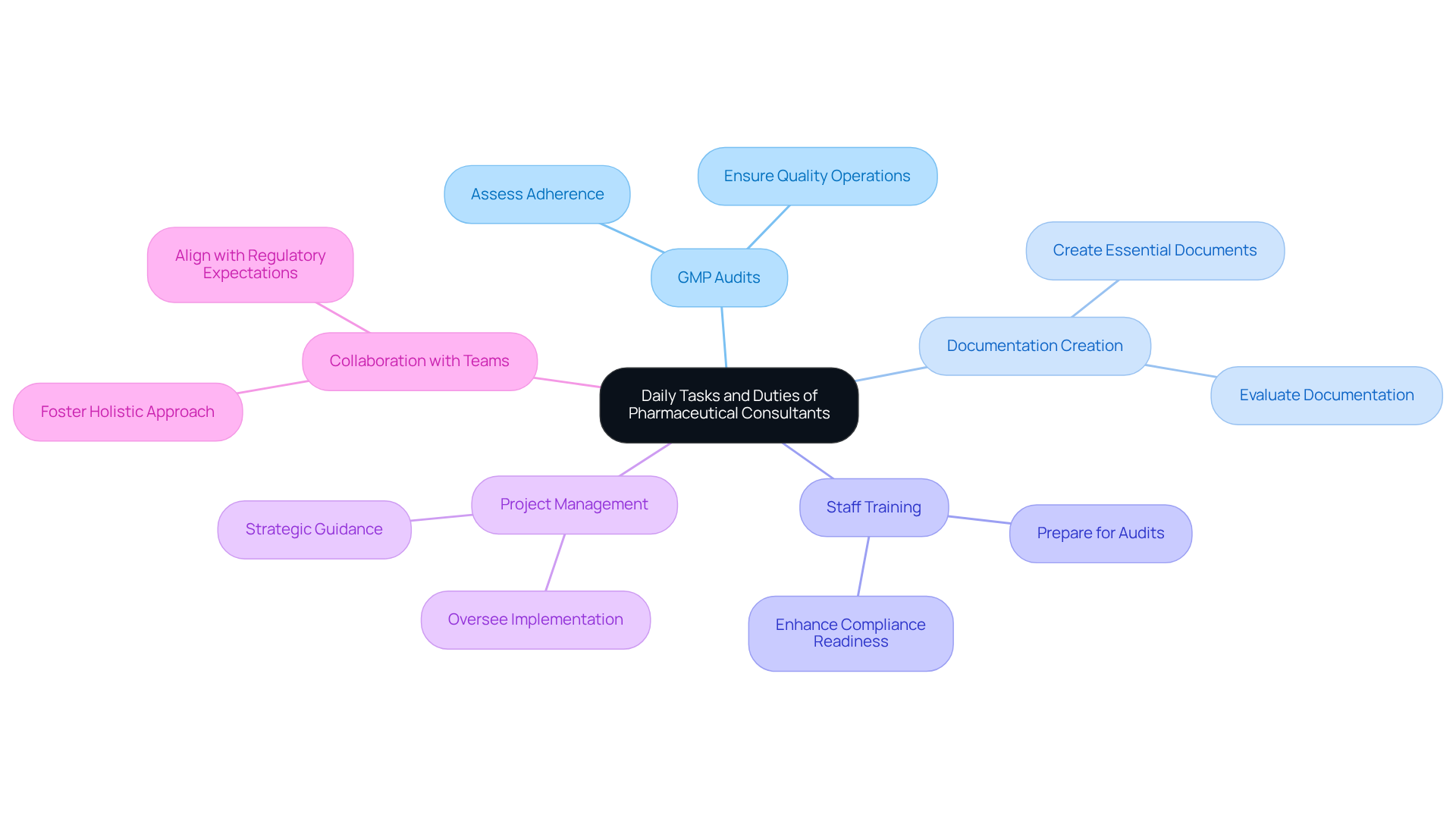
A pharmaceutical consultant plays a crucial role in addressing the specific needs of their clients through a variety of daily tasks. Central to their responsibilities is the performance of GMP audits, which assess adherence to established standards—an essential practice for maintaining high-quality operations. AVS Life Sciences stands out with its comprehensive GXP regulatory services, encompassing API & Drug Product CMOs and Contract Test Labs, ensuring that all materials comply with stringent regulatory requirements. Additionally, they create and evaluate essential documentation, reinforcing quality adherence throughout the drug development lifecycle.
Training staff on is another vital aspect of their role, equipping teams for audits and enhancing overall compliance readiness. AVS Life Sciences provides specialized training that empowers organizations with the knowledge necessary to effectively navigate complex compliance environments.
In conjunction with these tasks, advisors frequently take on project management responsibilities, overseeing the implementation of new processes or systems. They offer strategic guidance on best practices for clinical trial design and execution, ensuring that projects align with regulatory expectations. Collaboration with cross-functional teams is essential, fostering a holistic approach to product development that adheres to all necessary regulations.
Statistics indicate that efficient audits conducted by industry experts lead to improved compliance outcomes, with many organizations reporting no findings during inspections. This underscores the significant impact that experienced pharmaceutical consultants, such as those at AVS Life Sciences, have on ensuring compliance with Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR). By leveraging their expertise, pharmaceutical consultants play a pivotal role in navigating the complexities of regulatory frameworks, ultimately contributing to the success of their clients in the life sciences sector.
AVS Life Sciences, evidenced by its 80% repeat business rate, exemplifies the effectiveness of these professionals in delivering quality and regulatory solutions across a diverse clientele, ranging from startups to Fortune 100 companies.
Identify Essential Skills: Qualifications and Expertise Required for Pharmaceutical Consultants
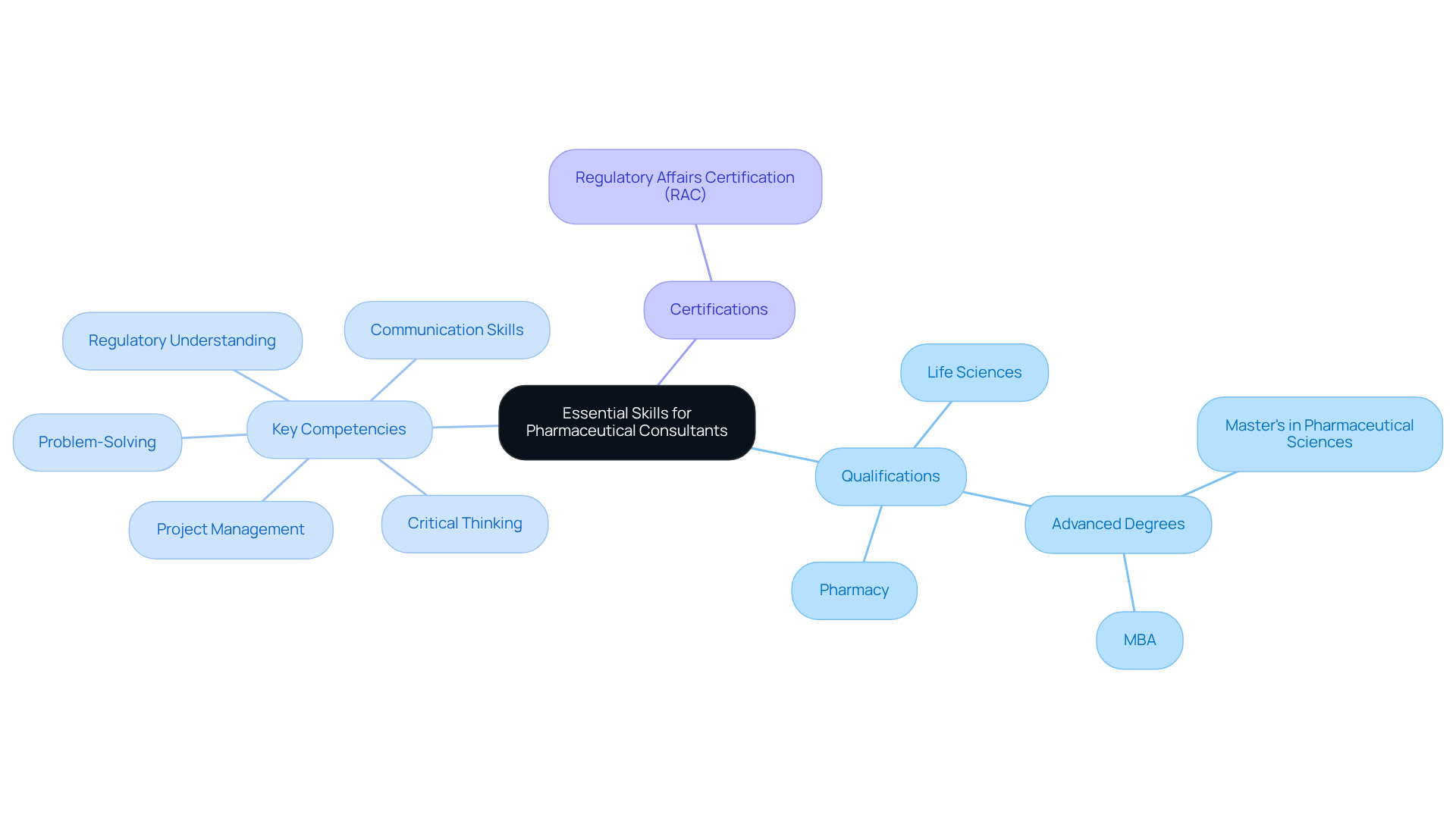
To excel as a pharmaceutical consultant, individuals must combine technical expertise with strong analytical and interpersonal skills. A solid educational foundation in life sciences, pharmacy, or a related discipline is crucial—often enhanced by advanced degrees such as a Master's in Pharmaceutical Sciences or an MBA. Certifications like the Regulatory Affairs Certification (RAC) are highly esteemed, as they deepen comprehension of oversight compliance and drug approval processes.
Key competencies encompass:
- A thorough understanding of regulatory frameworks
- Effective project management
- Exceptional communication skills, which are vital for articulating complex information to clients
Moreover, consultants should possess strong problem-solving abilities and critical thinking skills, enabling them to craft strategic recommendations that align with client objectives. As the pharmaceutical landscape evolves, having a pharmaceutical consultant with the necessary qualifications and skills remains essential for navigating the industry's complexities and ensuring successful outcomes.
To learn more about how these qualifications can be applied in practice, consider engaging with for tailored consulting solutions that meet your specific needs.
Conclusion
Pharmaceutical consultants are indispensable in the healthcare and drug development sectors, acting as expert advisors who navigate organizations through the intricate landscape of regulatory compliance, quality assurance, and operational efficiency. By harnessing their specialized knowledge and skills, these professionals not only refine product development strategies but also ensure adherence to essential industry standards, ultimately bolstering the safety and efficacy of pharmaceutical products.
Key responsibilities of pharmaceutical consultants include:
- Conducting GMP audits
- Formulating compliance policies
- Delivering training on quality management systems
Their expertise proves especially vital for startups and smaller enterprises that may lack internal resources, as these consultants streamline processes and cultivate a culture of quality assurance. The collaboration between pharmaceutical consultants and organizations, such as AVS Life Sciences, exemplifies the profound impact of expert guidance in navigating regulatory landscapes and enhancing overall product quality.
The significance of pharmaceutical consultants cannot be overstated; they are crucial in ensuring compliance and augmenting the operational capabilities of drug companies. As the pharmaceutical industry continues to evolve, organizations are urged to engage with qualified consultants to effectively navigate these challenges. By investing in expert guidance, companies can mitigate risks associated with regulatory compliance while driving innovation and achieving sustainable success in the competitive landscape of the life sciences sector.
Frequently Asked Questions
What is the primary role of a pharmaceutical consultant?
A pharmaceutical consultant serves as a specialized advisor, providing expert guidance to drug companies, healthcare organizations, and related agencies, particularly in drug development, regulatory compliance, and quality assurance.
What are the key responsibilities of a pharmaceutical consultant?
Their key responsibilities include navigating regulatory landscapes, ensuring adherence to Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR), and optimizing operational processes to enhance product development strategies.
How do pharmaceutical consultants contribute to quality assurance?
They play a crucial role in elevating quality assurance throughout drug development by helping organizations establish robust operational processes that enhance efficiency, minimize waste, and ultimately improve product quality.
Can you provide an example of a pharmaceutical consultant's impact?
An example is AVS Life Sciences's collaboration with a leading biotechnology company to upgrade their manufacturing facility from a Biosafety Level 1 GMP site to a Level 2 GMP facility, completed on schedule and within budget, showcasing a commitment to quality assurance and industry standards.
Why is regulatory compliance important in the pharmaceutical industry?
Regulatory compliance is crucial as it ensures adherence to safety regulations and government mandates, preventing costly errors and safeguarding product safety.
What expertise do pharmaceutical consultants bring to product development?
They possess proficiency in forecasting costs linked to the development of new medications and medical devices, enabling companies to manage investments wisely and prepare for financial implications.
How is the role of pharmaceutical consultants evolving?
As the pharmaceutical landscape continues to evolve, the role of pharmaceutical consultants, particularly those at AVS Life Sciences, remains essential for ensuring compliance and enhancing quality assurance for achieving success.
