What is a CAPA? Understanding Its Role in Quality Management

Overview
The article elucidates that a CAPA, or Corrective and Preventive Actions, represents a systematic approach that is vital for quality management in regulated sectors, particularly within pharmaceuticals and medical devices. This approach is designed to address existing quality issues while simultaneously preventing future occurrences. Such a dual focus is not merely beneficial; it is essential for compliance with regulatory standards and for fostering a culture of continuous improvement.
The article underscores the significance of effective CAPA processes, which are instrumental in enhancing operational efficiency and mitigating risks associated with product recalls.
Introduction
Understanding the intricacies of quality management is pivotal in sectors where safety and compliance are non-negotiable, such as pharmaceuticals and medical devices. Corrective and Preventive Actions (CAPA) serve as a cornerstone in these industries, offering a structured approach to identifying and resolving quality issues while preventing future occurrences.
However, as organizations strive to meet rigorous regulatory standards, the question arises: how can they effectively implement CAPA systems that not only address immediate concerns but also foster a culture of continuous improvement? This challenge presents an opportunity for organizations to enhance their compliance strategies and drive long-term success.
Define CAPA: The Essentials of Corrective and Preventive Actions
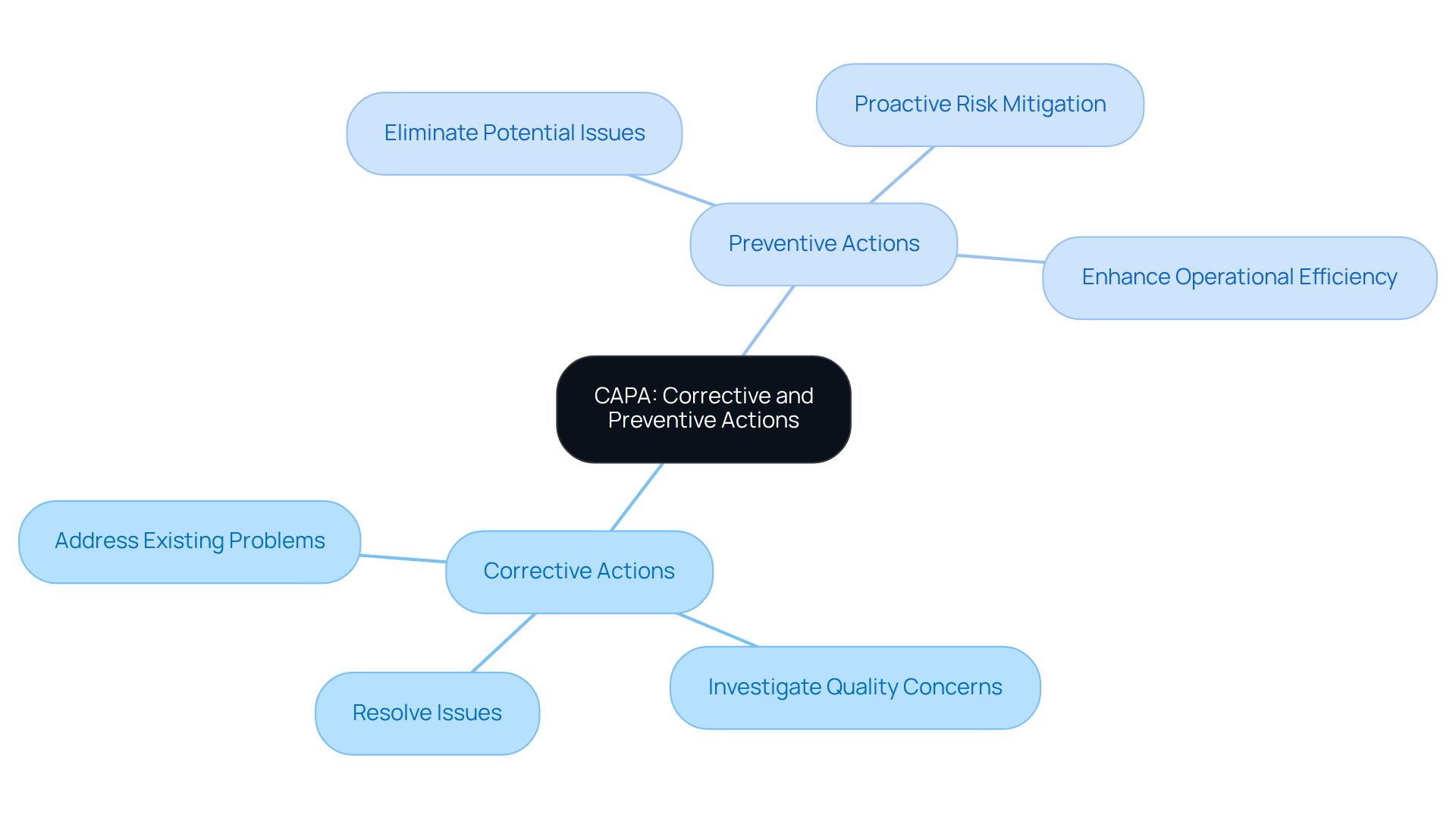
What is a capa? It stands for Corrective and Preventive Actions and represents a systematic approach utilized across various regulated sectors, particularly in pharmaceuticals and medical devices, to identify, investigate, and resolve quality concerns. The corrective element is dedicated to addressing existing problems, while the preventive aspect seeks to eliminate potential issues before they arise. This dual approach is vital for maintaining regulatory standards, such as Good Manufacturing Practices (GMP) and ISO standards, ensuring that organizations can deliver safe and effective products to the market.
At , we implement robust corrective and preventive action strategies that not only address urgent concerns but also proactively mitigate risks. Our extensive expertise in quality management and regulatory compliance guarantees that our clients meet industry standards while enhancing their operational efficiency. By engaging with AVS Life Sciences, organizations can navigate compliance challenges effectively and ensure the integrity of their products.
Contextualize CAPA: Importance in Regulatory Compliance and Quality Management
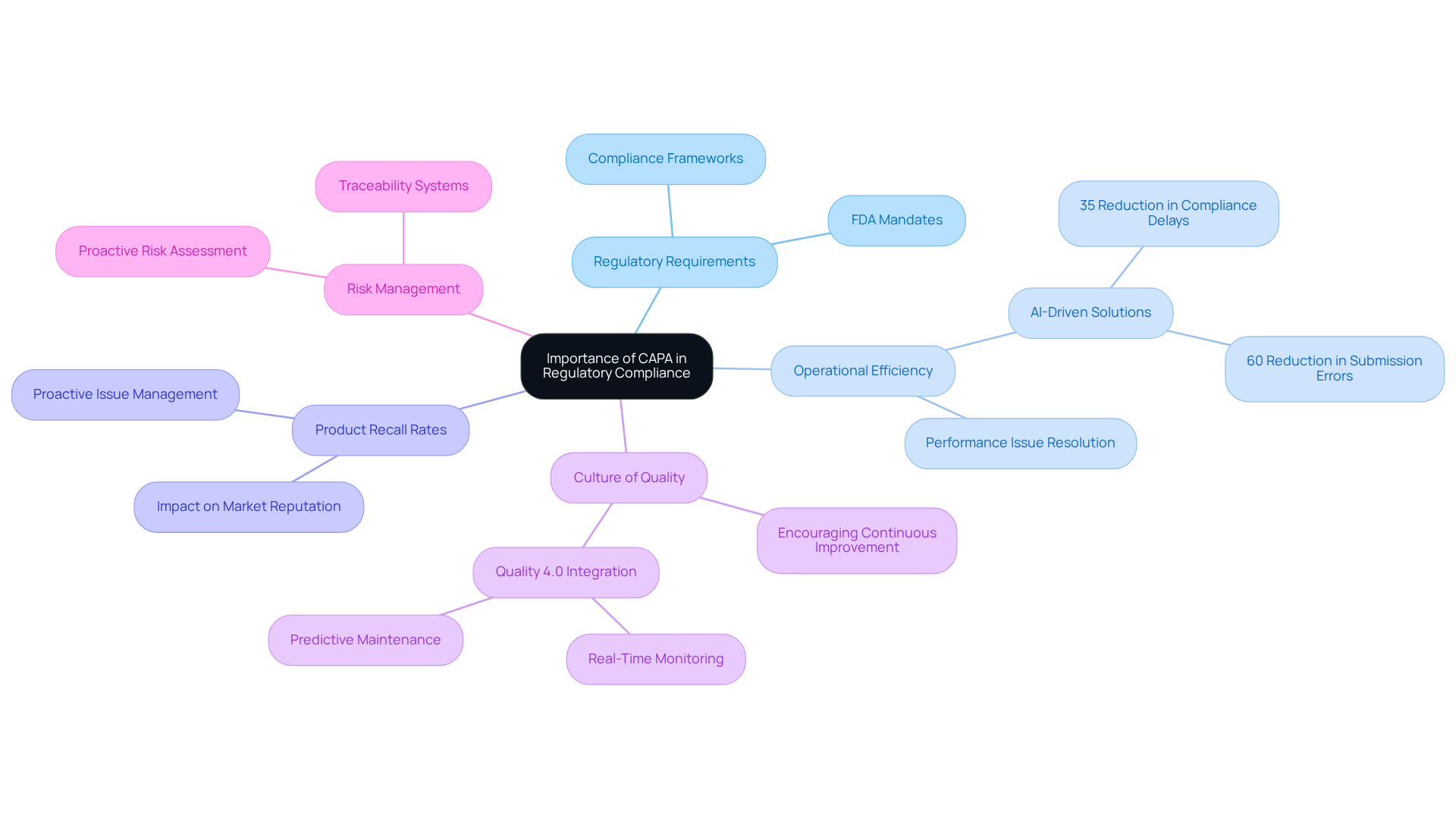
In the highly regulated life sciences sector, the implementation of Corrective and Preventive Action systems is not merely a best practice; it is a regulatory requirement. Regulatory agencies, such as the FDA, mandate organizations to implement efficient corrective and preventive action processes to tackle non-conformities and promote ongoing enhancement. By systematically identifying and resolving performance issues, organizations can significantly enhance operational efficiency and reduce the risk of product recalls. For instance, organizations that have implemented AI-driven solutions have noted a 35% decrease in regulation delays, underscoring the significance of prompt corrective and preventive actions.
Moreover, a robust corrective and preventive action process fosters a culture of quality within organizations, encouraging proactive risk management and continuous improvement. This is especially vital in upholding adherence to strict regulations, as directly impact product recall rates. By addressing potential issues before they escalate, organizations can not only safeguard their products but also uphold their reputation in the market. The incorporation of corrective and preventive actions into management frameworks is crucial for pharmaceutical firms seeking to navigate the complexities of regulatory compliance while ensuring the utmost standards of product excellence.
Trace the Origins: The Evolution of CAPA in Life Sciences
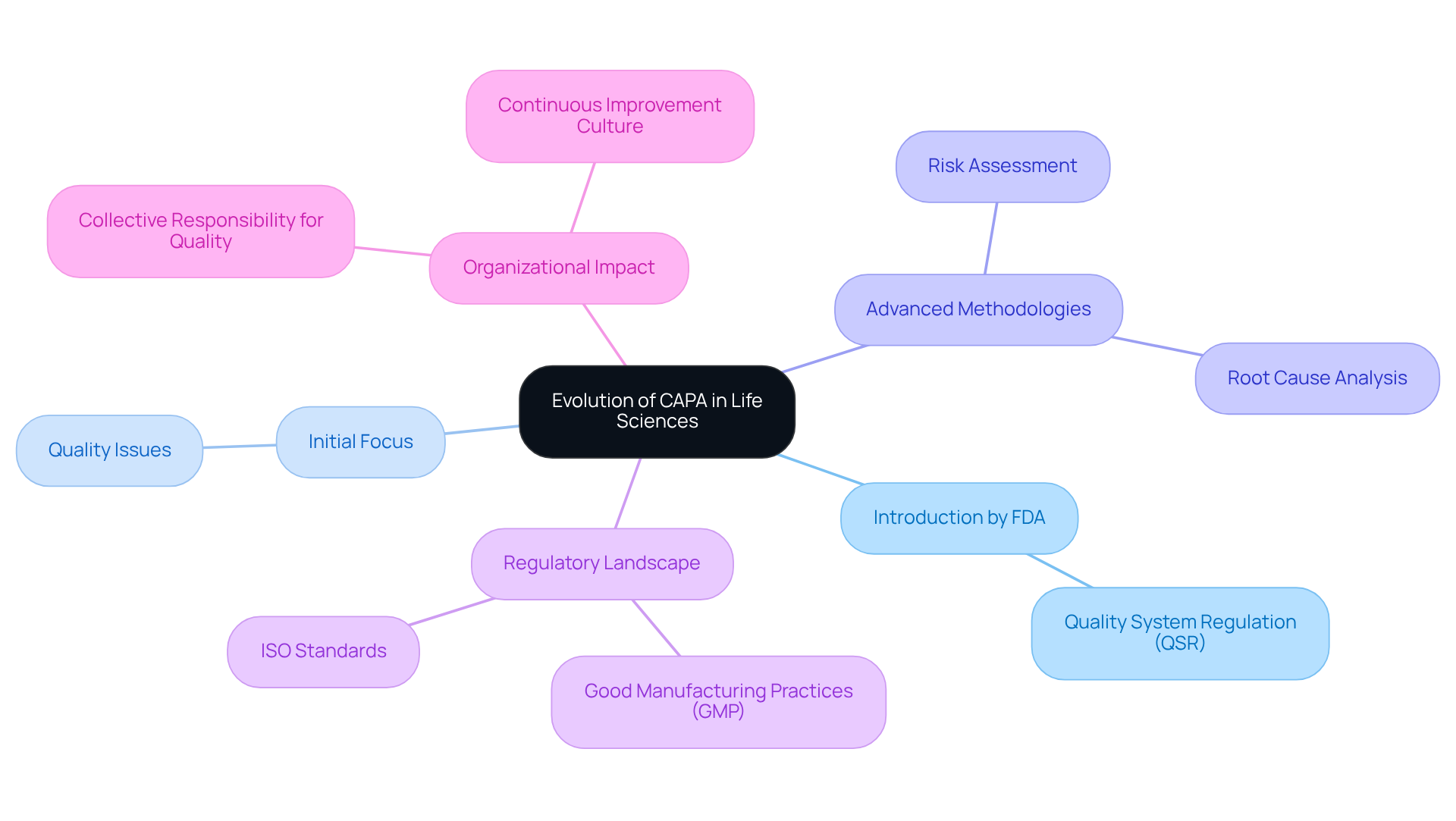
Since its formal introduction by the FDA in the 1990s as part of the Quality System Regulation (QSR), what is a capa has evolved significantly. Initially focused on addressing urgent quality issues, this system has developed into a robust framework that highlights what is a capa in terms of preventive actions and continuous improvement.
Organizations now leverage advanced methodologies, including root cause analysis and risk assessment, to better understand what is a capa and refine their processes. This transformation underscores the sector's commitment to product safety and effectiveness within a complex regulatory landscape, which includes adherence to (GMP) and ISO standards.
The question of what is a capa has become increasingly relevant as its integration into management systems reflects a collective responsibility for excellence across all organizational levels. Consequently, businesses are better equipped to navigate regulatory challenges, ensuring that quality is not merely a departmental task but a fundamental aspect of their operational philosophy.
Furthermore, understanding the implications of privacy policies is crucial, as these policies play a vital role in the broader regulatory framework, ensuring that organizations comply with standards while managing sensitive information.
Establishing objectives and standards prior to gathering data for verification and validation is essential for evaluating effectiveness and efficiency, ultimately fostering a culture of excellence within organizations.
Identify Key Components: Characteristics of an Effective CAPA Plan
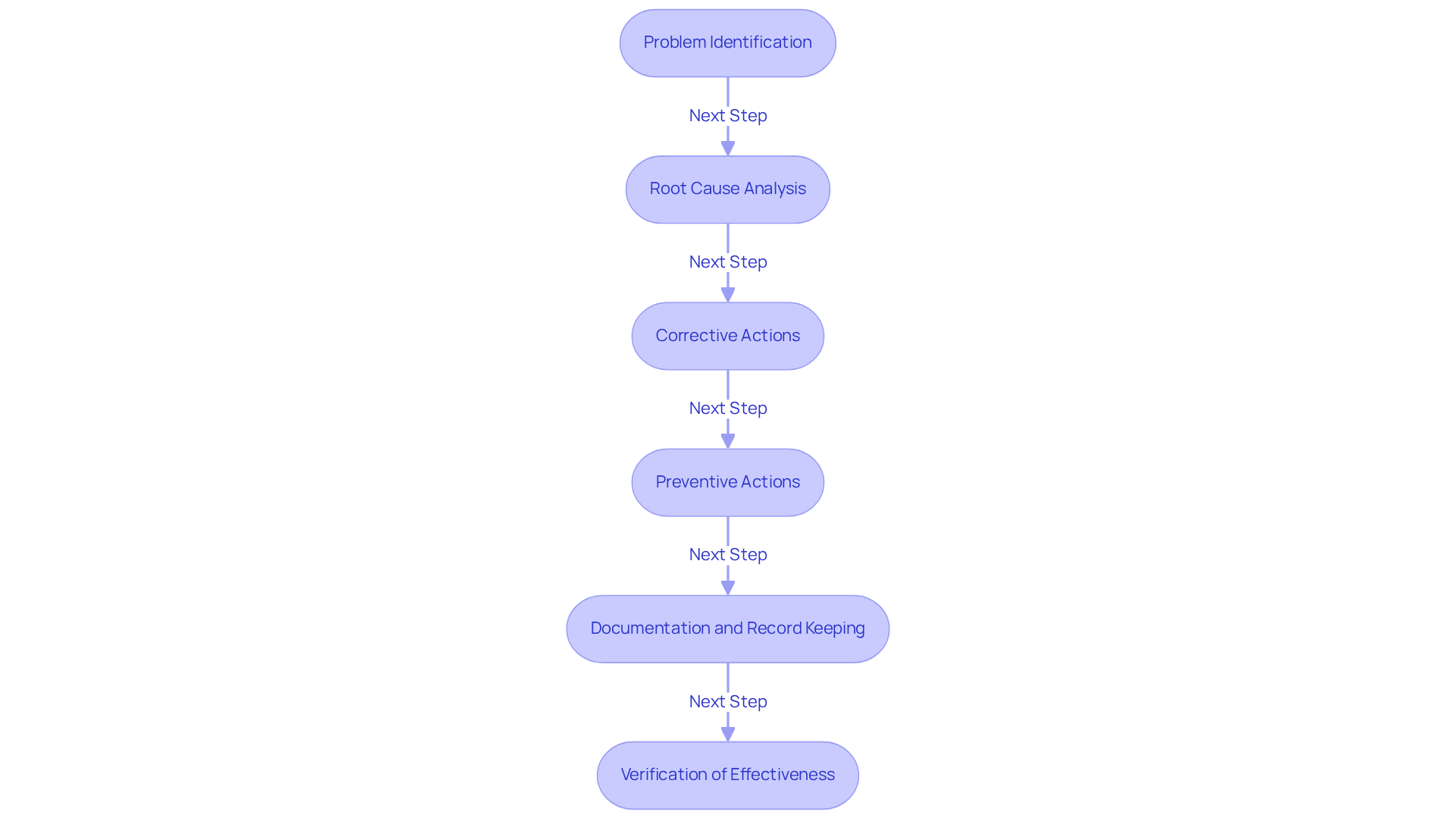
An effective CAPA plan includes several essential components that clarify what is a CAPA, ensuring quality management and regulatory compliance.
- Problem Identification: Clearly defining the challenge at hand is crucial for effective resolution. This step often involves rigorous monitoring mechanisms to detect deviations, defects, or compliance problems.
- Root Cause Analysis: Investigating the underlying causes of the problem is vital to prevent recurrence. Methods such as the 5 Whys and Fishbone Diagram are frequently employed to pinpoint root causes, ensuring that corrective actions address the actual problems rather than merely symptoms.
- Corrective Actions: Implementing measures to address identified issues is necessary for immediate resolution. This step should be data-driven; notably, 75% of FDA warning letters cite shortcomings in the , underscoring the significance of efficient remedial measures.
- Preventive Actions: Establishing processes to mitigate the risk of future occurrences is essential for long-term assurance of standards. A well-structured corrective and preventive action process not only resolves current issues but also enhances a robust quality system that safeguards a company's reputation and financial outcomes.
- Documentation and Record Keeping: Maintaining thorough records of all CAPA activities ensures traceability and compliance with regulatory bodies. Complete documentation is crucial for audits and inspections, providing transparency and accountability.
- Verification of Effectiveness: Evaluating the efficacy of executed measures is essential to confirm that concerns have been adequately addressed. This includes follow-up audits and control checks to ensure that corrective and preventive measures function as intended.
By incorporating these components, organizations can establish a robust system that exemplifies what is a CAPA, resolving current quality issues while fostering a culture of continuous improvement and ultimately enhancing regulatory compliance and operational effectiveness.
Conclusion
The concept of Corrective and Preventive Actions (CAPA) stands as a cornerstone in quality management, particularly within the highly regulated life sciences sector. By systematically addressing existing problems and preventing potential issues, CAPA ensures that organizations maintain compliance with stringent regulatory standards while delivering safe and effective products. This dual approach not only safeguards product integrity but also fosters a culture of quality and continuous improvement within organizations.
Throughout this article, we have explored key insights into the importance of CAPA, including its essential components:
- Problem identification
- Root cause analysis
- Implementation of corrective and preventive actions
The historical evolution of CAPA since its introduction by the FDA highlights its growing significance in today's regulatory landscape. Organizations are increasingly leveraging advanced methodologies to enhance their processes and compliance efforts. The emphasis on documentation and verification further underscores the necessity of a robust CAPA system in mitigating risks and ensuring operational excellence.
Ultimately, the integration of effective CAPA strategies is vital for organizations aiming to navigate the complexities of regulatory compliance while upholding the highest standards of quality. By prioritizing CAPA within their operational frameworks, businesses not only protect their reputation and financial outcomes but also contribute to a broader culture of safety and excellence in the industry. Embracing these practices can lead to significant improvements in product quality and regulatory adherence, paving the way for sustainable success in the competitive landscape of life sciences.
Frequently Asked Questions
What does CAPA stand for?
CAPA stands for Corrective and Preventive Actions.
What is the purpose of CAPA?
CAPA represents a systematic approach used to identify, investigate, and resolve quality concerns in regulated sectors, particularly in pharmaceuticals and medical devices.
What is the difference between corrective and preventive actions in CAPA?
The corrective element focuses on addressing existing problems, while the preventive aspect aims to eliminate potential issues before they arise.
Why is CAPA important in regulated industries?
CAPA is vital for maintaining regulatory standards, such as Good Manufacturing Practices (GMP) and ISO standards, ensuring that organizations deliver safe and effective products to the market.
How does AVS Life Sciences implement CAPA strategies?
AVS Life Sciences implements robust CAPA strategies that address urgent concerns and proactively mitigate risks, ensuring compliance and enhancing operational efficiency.
What expertise does AVS Life Sciences offer in relation to CAPA?
AVS Life Sciences offers extensive expertise in quality management and regulatory compliance, helping clients meet industry standards and navigate compliance challenges effectively.
