What is a 503B Pharmacy? Key Characteristics and Compliance Insights

Overview
A 503B pharmacy serves as an outsourcing center that compounds sterile medications in bulk, eliminating the necessity for individual patient prescriptions. This capability ensures a consistent supply for healthcare providers, particularly crucial during periods of drug shortages. Key characteristics of these facilities include:
- A strict adherence to Good Manufacturing Practices (GMP)
- Oversight by the FDA
These factors collectively guarantee the maintenance of high safety and quality standards essential for patient care. Such compliance not only addresses immediate supply challenges but also reinforces the integrity of patient treatment protocols.
Introduction
The landscape of pharmaceutical care is evolving, particularly with the emergence of specialized compounding facilities known as 503B pharmacies. These establishments play a crucial role in addressing the increasing demand for compounded medications, especially in the face of rising drug shortages—reported to have surged by 38% between 2021 and 2023. By enabling the production of bulk sterile medications without the need for individual prescriptions, 503B pharmacies ensure that healthcare providers have timely access to essential treatments.
However, as these facilities navigate a complex regulatory environment, questions arise:
- How do they maintain compliance with stringent standards?
- What distinguishes them from traditional compounding pharmacies?
Exploring these dynamics reveals the critical importance of 503B pharmacies in the modern healthcare system.
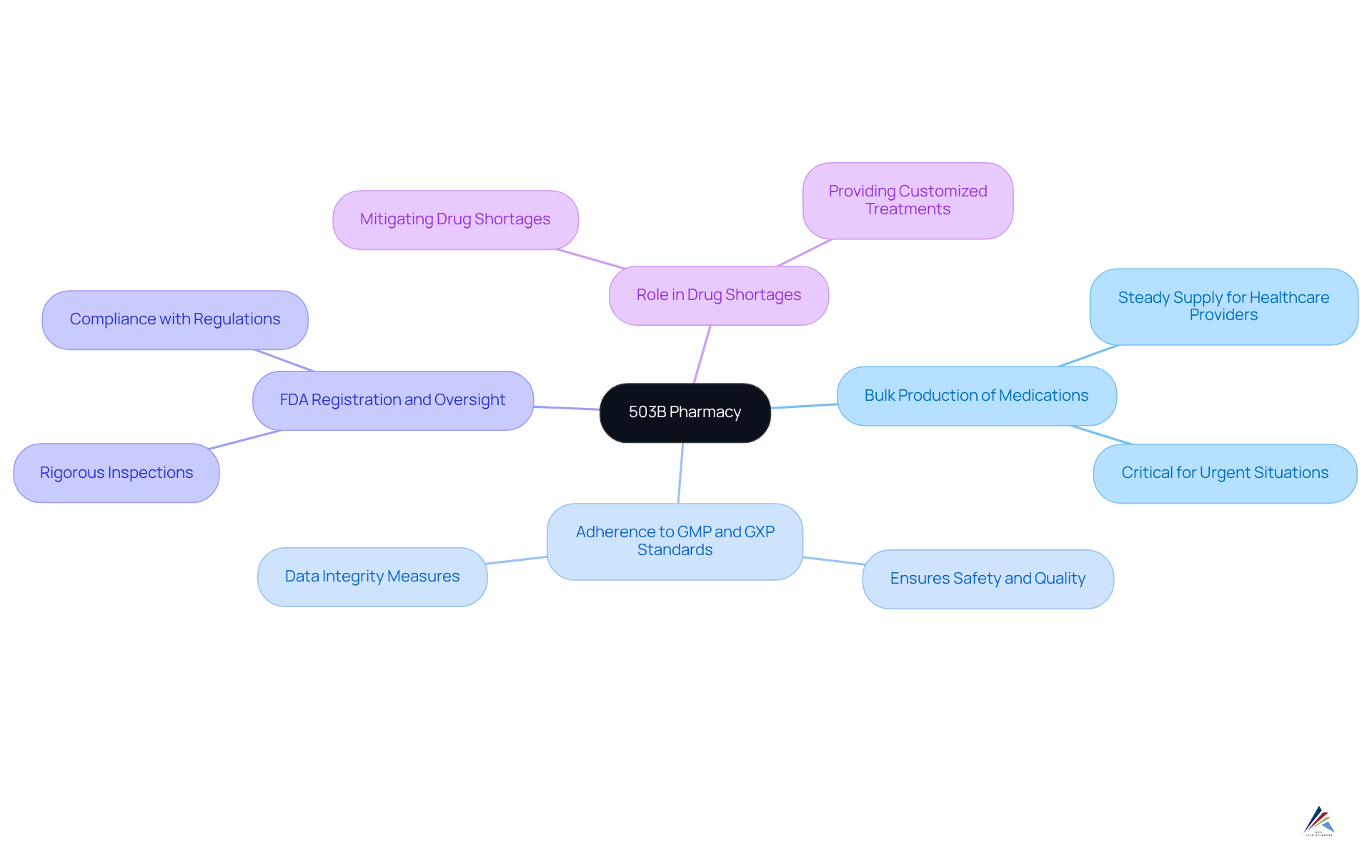
Define 503B Pharmacy: Key Characteristics and Functions
An outsourcing center established under the Drug Quality and Security Act (DQSA) of 2013 is dedicated to compounding sterile medications for human use. Unlike conventional drugstores, certain facilities possess the capability to produce larger volumes of compounded treatments without requiring prescriptions tailored to individual patients. This capability is essential for healthcare facilities and providers, particularly during urgent situations where immediate access to compounded drugs is critical.
Key characteristics of 503B pharmacies include:
- The ability to produce bulk quantities of compounded medications, ensuring a steady supply for healthcare providers.
- Strict adherence to Good Manufacturing Practices (GMP) and GXP standards, which ensure that compounded products meet high safety and quality standards, including data integrity measures.
- Mandatory registration with the FDA, subjecting these establishments to rigorous inspections and oversight, ensuring compliance with FDA regulations and GXP standards.
What is a 503B pharmacy, and how do these pharmacies play a pivotal role in enhancing healthcare accessibility by supplying essential prescriptions that may be unavailable in the market? A significant number of establishments provide a substantial portion of healthcare centers with customized treatments, addressing critical gaps in therapeutic alternatives. Their operations exemplify successful compliance with regulatory standards, ensuring that patients receive safe and effective medications tailored to their specific needs.
Notably, the FDA has reported a 38% increase in drug shortages between 2021 and 2023, underscoring the importance of specialized compounding facilities in mitigating these shortages. Furthermore, with fewer than 80 compounding establishments receiving FDA approval, the exclusivity and regulatory rigor surrounding these facilities bolster their credibility. As noted by Novo Nordisk, compounded treatments often represent a more cost-effective or accessible option, particularly for those who cannot tolerate commercial formulations. Additionally, the Olympia Compounding Center, the sole outsourcing establishment in Central Florida, exemplifies the operational capabilities and adherence of specialized compounding services. These facilities are also the exclusive compounding dispensaries authorized to supply office-use medications, as designated by the FDA, further highlighting their unique role in the healthcare landscape. This context aligns with , demonstrating how effective quality control and compliance measures can lead to successful outcomes in the biotechnology sector.
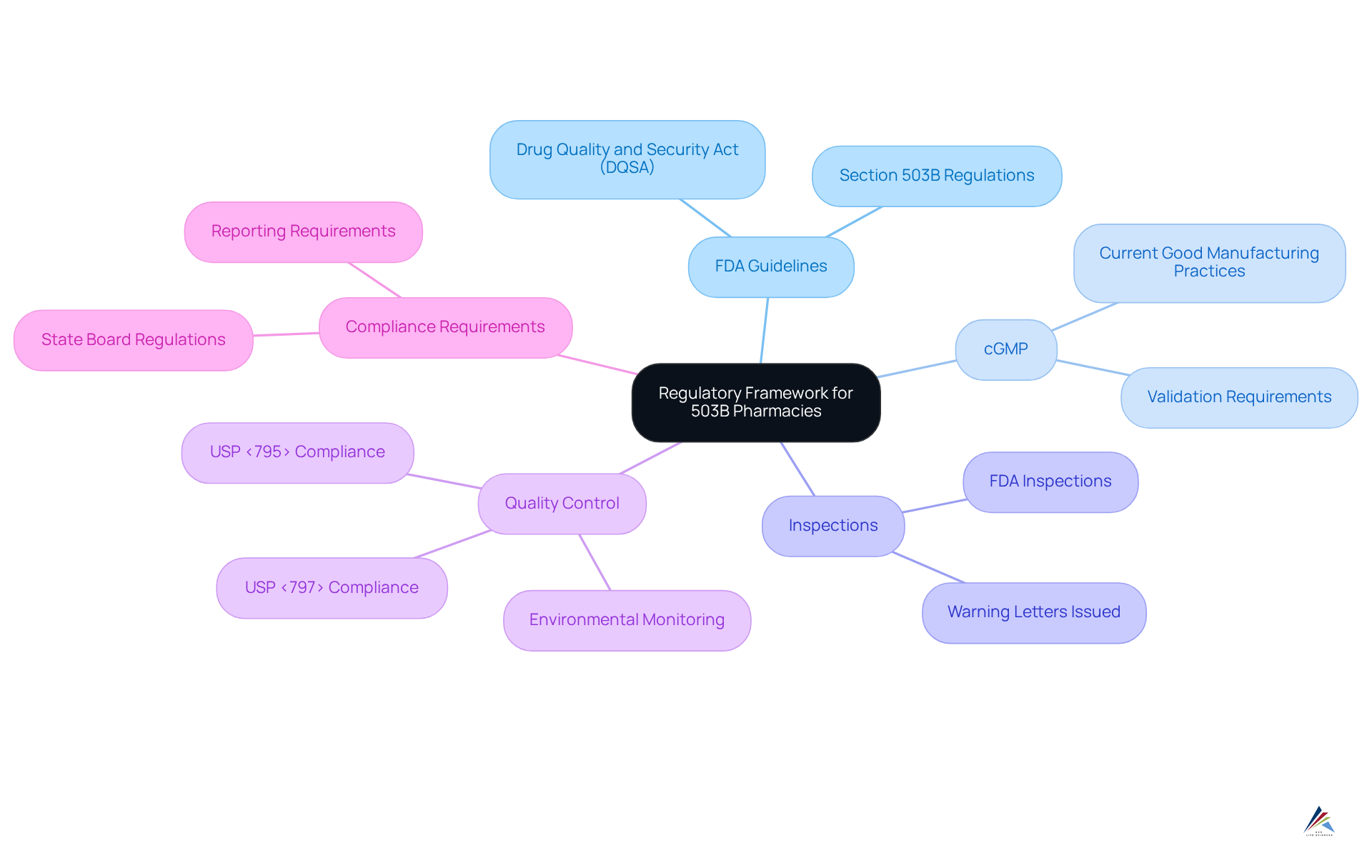
Examine Regulatory Framework: Compliance and Oversight of 503B Pharmacies
The regulatory framework governing compounding facilities is fundamentally established by the FDA, which mandates strict adherence to current Good Manufacturing Practices (cGMP) as delineated in the Drug Quality and Security Act (DQSA). These facilities are required to comply with rigorous guidelines regarding facility design, equipment, personnel qualifications, and quality control processes. In 2025 alone, the FDA conducted over 400 inspections of compounding facilities, resulting in more than 150 warning letters aimed at ensuring compliance with these essential standards.
Routine evaluations are crucial for confirming adherence and safeguarding public health, as they compel establishments to report negative incidents while maintaining comprehensive documentation of their compounding practices. Furthermore, when discussing what is a 503b pharmacy, it is essential to note that these pharmacies must comply with USP <795> and <797>, in addition to state board regulations and 21 CFR Part 210 and 211 (cGMP). This oversight is vital for ensuring that compounded treatments are both safe and effective for patient use.
The regulatory landscape is continuously evolving, with ongoing discussions focused on enhancing oversight to address emerging challenges within the compounding industry. As emphasized by FDA officials, maintaining compliance with these standards is indispensable for the integrity of compounded medications. Moreover, compounding facilities are mandated to establish a separate quality department to ensure thorough supervision of their operations, including the execution of environmental monitoring programs essential for compliance.
To navigate these complex regulations, in quality management and regulatory compliance, offering essential guidance for compliance officers. Additionally, user manuals are available to assist in understanding and effectively implementing these regulatory requirements. By engaging with AVS Life Sciences, facilities can enhance their compliance strategies, ensuring that they meet the evolving demands of the industry.
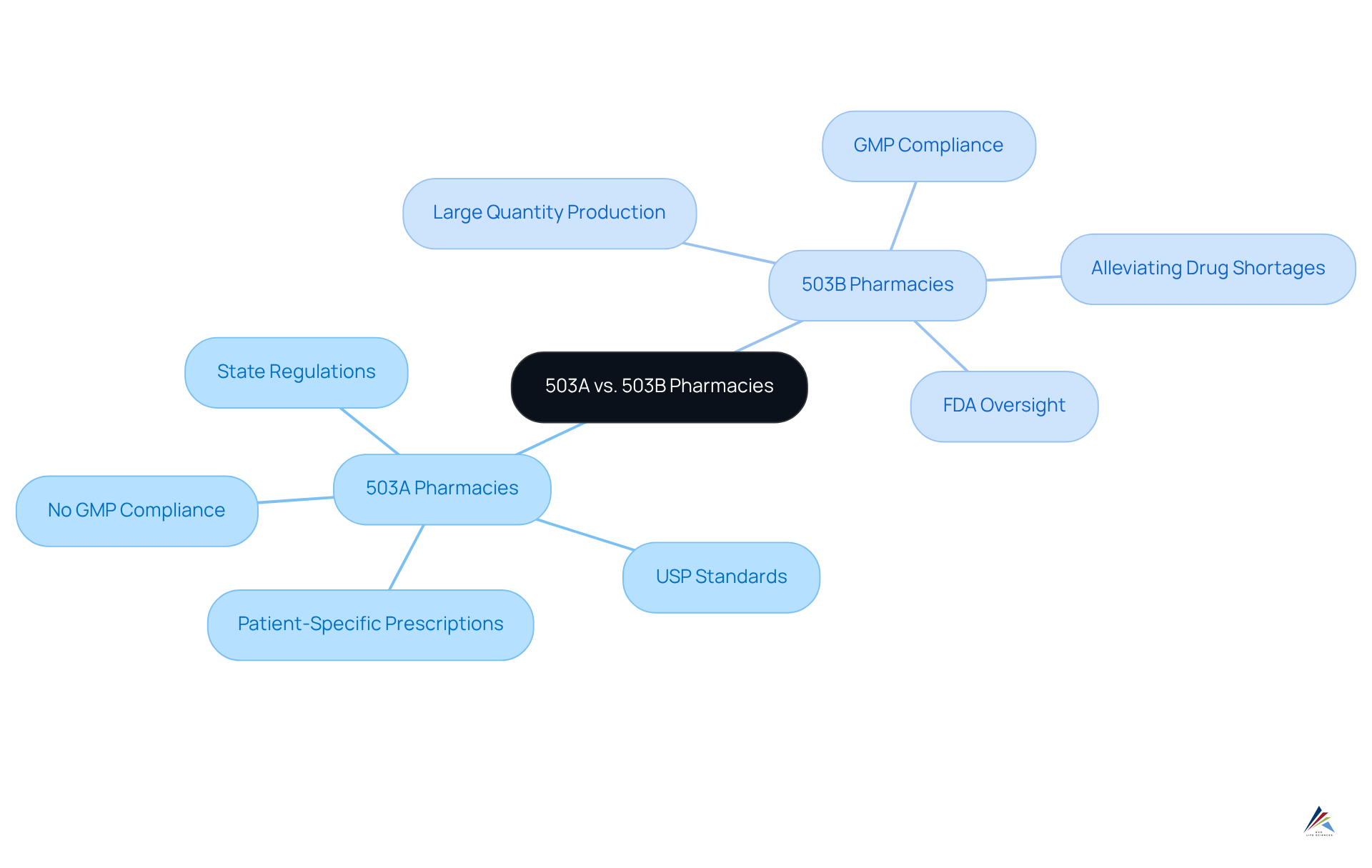
Differentiate 503A and 503B Pharmacies: Understanding the Distinctions
In the compounding landscape, understanding what is a 503B pharmacy is essential as 503A and 503B establishments play distinct roles. A 503A compounding establishment is dedicated to creating treatments tailored to specific patient prescriptions. These drugstores operate under state regulations and must comply with United States Pharmacopeia (USP) standards; however, they are not mandated to follow Good Manufacturing Practices (GMP). In contrast, certain outsourcing establishments, often referred to when discussing what is a 503B pharmacy, produce substances in large quantities without the necessity of prescriptions designed for individual patients. These establishments are required to adhere to GMP and are subject to rigorous FDA oversight.
This distinction is crucial, as it influences the scope of practice, regulatory compliance, and the types of substances that can be compounded. For instance, licensed establishments have the capacity to generate substantial quantities of compounded products, which aids in alleviating drug shortages and reducing costs for healthcare providers. As we approach 2025, the regulatory landscape continues to evolve, with 503A facilities undergoing increased scrutiny to ensure compliance with current Good Manufacturing Practices (cGMP), while understanding what is a 503B pharmacy requires strict adherence to USP guidelines and state regulations.
Understanding these differences is essential for healthcare providers and patients navigating medication options, as it directly impacts the . The FDA has underscored that 503A pharmacies must comply with USP <795> and <797> guidelines, while 503B pharmacies are required to validate every process in accordance with cGMP, thereby ensuring high-quality standards in their operations.
Conclusion
Understanding the role of 503B pharmacies is crucial for grasping how they enhance healthcare delivery through the compounding of sterile medications. These specialized outsourcing facilities not only produce bulk quantities of compounded drugs but also adhere to stringent regulatory standards, ensuring the safety and quality of the medications they provide. The distinct capabilities of 503B pharmacies set them apart from traditional pharmacies, highlighting their importance in addressing urgent healthcare needs.
The article delves into the key characteristics of 503B pharmacies, including their compliance with Good Manufacturing Practices (GMP) and FDA regulations, which are vital for maintaining high-quality standards. It emphasizes the growing significance of these facilities in mitigating drug shortages, particularly in light of the reported increase in drug shortages from 2021 to 2023. Furthermore, the differentiation between 503A and 503B pharmacies underscores the unique operational frameworks and regulatory requirements that govern their practices, impacting the availability and efficacy of compounded medications.
In conclusion, the insights presented reveal the indispensable role that 503B pharmacies play in the modern healthcare landscape. As the regulatory environment continues to evolve, it is essential for healthcare providers and patients to remain informed about their options in compounded medications. Engaging with these specialized facilities not only ensures access to critical treatments but also supports the ongoing efforts to improve compliance and quality in the pharmaceutical industry. Embracing the advancements in compounding practices and understanding their implications can lead to better healthcare outcomes for all.
Frequently Asked Questions
What is a 503B pharmacy?
A 503B pharmacy is an outsourcing center established under the Drug Quality and Security Act (DQSA) of 2013, dedicated to compounding sterile medications for human use, capable of producing larger volumes of compounded treatments without requiring individual patient prescriptions.
What are the key characteristics of 503B pharmacies?
Key characteristics include the ability to produce bulk quantities of compounded medications, strict adherence to Good Manufacturing Practices (GMP) and GXP standards, and mandatory registration with the FDA, which subjects these pharmacies to rigorous inspections and oversight.
How do 503B pharmacies enhance healthcare accessibility?
503B pharmacies enhance healthcare accessibility by supplying essential compounded prescriptions that may be unavailable in the market, providing customized treatments that address critical gaps in therapeutic alternatives.
What is the significance of the increase in drug shortages reported by the FDA?
The FDA reported a 38% increase in drug shortages between 2021 and 2023, highlighting the vital role of specialized compounding facilities in mitigating these shortages and ensuring that healthcare providers have access to necessary medications.
How many compounding establishments have received FDA approval?
Fewer than 80 compounding establishments have received FDA approval, indicating the exclusivity and regulatory rigor surrounding these facilities.
What advantages do compounded treatments offer compared to commercial formulations?
Compounded treatments often represent a more cost-effective or accessible option for patients, particularly for those who cannot tolerate commercial formulations.
Can 503B pharmacies supply office-use medications?
Yes, 503B pharmacies are the exclusive compounding dispensaries authorized to supply office-use medications as designated by the FDA.
What example is mentioned to illustrate the capabilities of specialized compounding services?
The Olympia Compounding Center is mentioned as the sole outsourcing establishment in Central Florida, exemplifying the operational capabilities and adherence to regulatory standards of specialized compounding services.
