What Does GxP Stand For? Key Insights for Compliance Officers

Overview
GxP, an acronym for 'Good Practice,' refers to a comprehensive set of regulations and guidelines, including Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), and Good Laboratory Practices (GLP). These standards are essential for ensuring quality and integrity within the life sciences sector. This article underscores the pivotal role of GxP in maintaining product quality and compliance, highlighting that strict adherence to these standards is not merely recommended but crucial for obtaining regulatory approval and protecting public health.
Introduction
Understanding the intricacies of GxP—an acronym for 'Good Practice'—is essential for compliance officers navigating the complex landscape of the life sciences sector. These guidelines, encompassing Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), and Good Laboratory Practices (GLP), represent not merely regulatory requirements; they form critical frameworks that ensure product quality and safety.
As the industry faces increasing scrutiny and evolving regulations, organizations must confront the pressing question: how can they effectively implement GxP standards to not only comply but also thrive in this competitive environment?
By addressing compliance challenges head-on and exploring detailed solutions, organizations can transform regulatory adherence into a strategic advantage, fostering a culture of excellence and innovation.
Define GxP: Understanding Good Practice Standards
GxP, which is an acronym for 'Good Practice,' is often discussed in relation to what does GxP stand for, as it represents a comprehensive set of regulations and guidelines that govern the quality and integrity of products within the life sciences sector. This term encompasses critical guidelines, including:
Each of these practices is designed to ensure that products are consistently manufactured and managed according to established quality standards, thereby safeguarding public health and ensuring the efficacy of pharmaceuticals, biotechnology products, and medical devices.
The impact of GxP guidelines on product quality in the pharmaceutical industry is profound. These standards serve as the foundation for regulatory compliance, compelling organizations to adhere to stringent quality assurance protocols, which include meticulous documentation practices and effective internal auditing methods. Compliance officers must understand what does GxP stand for, as it represents not merely a collection of guidelines but a crucial framework that supports the operational integrity of their organizations.
AVS Life Sciences exemplifies the effective implementation of GxP principles, particularly highlighted through a transformative case study involving a leading biotechnology firm. AVS assisted this client in upgrading their manufacturing environment from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, successfully completing the project on time and within budget. This collaboration not only ensured compliance with rigorous regulatory standards but also enhanced the client's quality assurance processes, underscoring the essential role of GxP in preserving product integrity.
Expert insights underscore the importance of Good Practice guidelines in the pharmaceutical sector, especially as the industry faces increased scrutiny from regulatory bodies. By 2025, the significance of GxP in life sciences regulations will continue to rise, necessitating that companies adopt these guidelines effectively to maintain their competitive edge and ensure patient safety.
The successful in pharmaceutical companies requires a steadfast commitment to continuous improvement and adherence to best practices. Organizations that prioritize GxP compliance not only elevate their quality but also cultivate trust with stakeholders, ultimately leading to superior outcomes in the highly regulated life sciences landscape.
Contextualize GxP: Importance in Life Sciences Compliance
In the life sciences field, knowing what GxP stands for is crucial for guaranteeing that items comply with strict safety and efficacy criteria before reaching consumers. Compliance with GxP is not merely a regulatory obligation; it signifies a commitment to quality that fosters trust among stakeholders, including regulatory bodies, healthcare professionals, and patients, and raises the question of what GxP stands for in this context.
Organizations adhering to GxP standards effectively mitigate risks associated with recalls, legal liabilities, and reputational damage, highlighting the importance of understanding what GxP stands for. For instance, firms such as White Raven achieved [Good Manufacturing Practice (GMP)](https://avslifesciences.com/industry-news/cders-quality-management-maturity-program) certification in just 18 months, demonstrating how swift adherence can enhance market credibility.
Furthermore, companies aiming to introduce new offerings to the market must understand what GxP stands for, as [GxP compliance](https://kiteworks.com/risk-compliance-glossary/gxp) is often a prerequisite for obtaining regulatory approvals. The emphasis on what GxP stands for reflects the industry's dedication to maintaining throughout the lifecycle of products, which ultimately leads to improved patient safety and satisfaction.
As the biologics market continues to expand, projected to grow at a compound annual growth rate of 15% until 2027, understanding what GxP stands for will become increasingly important, reinforcing its role in facilitating market access and ensuring the integrity of offerings.
Explore GxP Variations: What the 'x' Represents
The 'x' in GxP signifies a variable that encompasses various practices tailored to specific facets of the life sciences industry, prompting the question, what does gxp stand for? Good Manufacturing Practices (GMP) concentrate on manufacturing processes, ensuring that products are consistently produced and controlled in accordance with stringent quality standards. In contrast, Good Clinical Practices (GCP) emphasize the ethical and scientific quality of clinical trials, safeguarding the rights and well-being of participants. Good Laboratory Practices (GLP) focus on laboratory operations, ensuring that data generated is reliable and reproducible. For regulation officers, understanding these distinctions is crucial, as each guideline offers unique requirements and implications for their organizations.
Current trends indicate a growing emphasis on robust Chemistry, Manufacturing, and Controls (CMC) practices, which are essential for regulatory adherence and innovation. Companies prioritizing CMC are better positioned for successful market entry and expedited development timelines. Organizations implementing have reported significant reductions in adverse events during clinical trials, thereby enhancing patient safety and fostering trust in new medical therapies.
In clinical trials, adherence to GCP is paramount. AstraZeneca's application of GCP principles has led to improved participant safety and data integrity, ultimately resulting in cost savings of $250,000 by deferring expenses related to yield loss and impurity increases. Such examples underscore the importance of adhering to GCP guidelines to ensure ethical conduct and scientific rigor in clinical research.
Grasping the subtleties of GMP, GCP, and GLP is essential for regulatory officers maneuvering through the intricacies of regulatory environments in 2025 and beyond. As the life sciences field progresses, keeping abreast of these methods, including Excellent Documentation Practices and Operating Procedures (SOPs), will enable organizations to uphold high levels of quality and adherence. Firms such as AVS Life Sciences, focusing on assisting pharmaceutical producers with facility enhancements—like moving from a Biosafety Level 1 to a Level 2 GMP facility—illustrate the essential function of expert solutions in attaining regulatory standards and improving operational capabilities.

Identify GxP Guidelines: Regulatory Bodies and Compliance Oversight
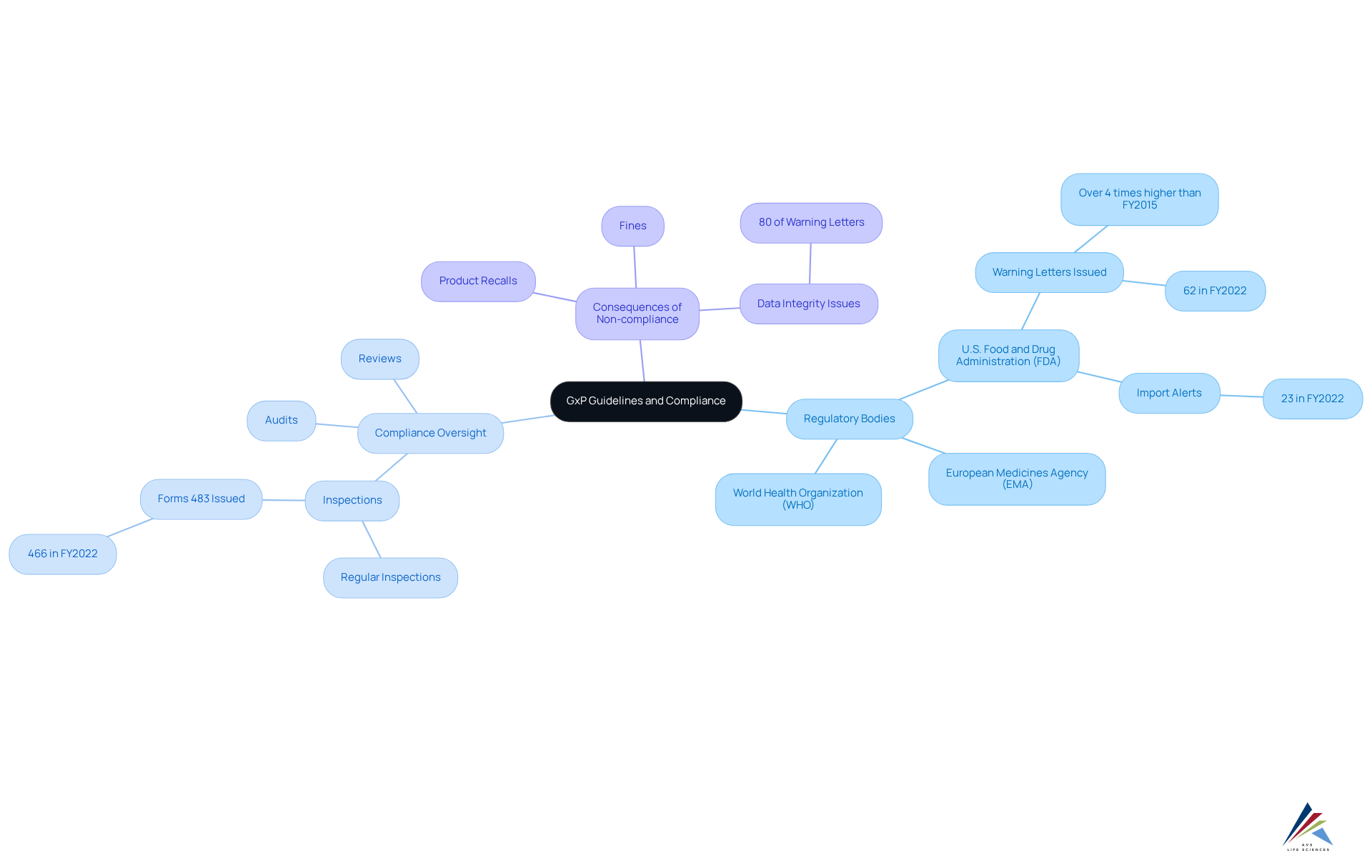
GxP guidelines are enforced by prominent regulatory bodies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO). These organizations establish the criteria that companies must adhere to in order to ensure the quality and safety of their offerings. Compliance oversight is a critical process that encompasses regular inspections, audits, and reviews to verify adherence to GxP guidelines.
For instance, in the fiscal year 2022, the FDA issued 62 warning letters and 23 import alerts concerning drugs, underscoring the agency's proactive role in overseeing adherence. Non-compliance can lead to severe repercussions, including substantial fines, product recalls, and loss of market access. Alarmingly, almost 80% of FDA warning letters reference data integrity problems, highlighting the significance of robust regulatory systems.
Therefore, it is essential for compliance officers to remain vigilant and informed about the evolving regulatory landscape. By ensuring that their organizations consistently meet the standards of what does gxp stand for, they can mitigate risks and maintain market integrity. The imperative for compliance is clear: is not just beneficial but necessary for sustaining operational credibility and success.
Conclusion
Understanding GxP is essential for compliance officers operating within the life sciences sector, as it represents a framework of Good Practice standards that ensures product quality and regulatory adherence. This term encapsulates various guidelines, including:
- Good Manufacturing Practices (GMP)
- Good Clinical Practices (GCP)
- Good Laboratory Practices (GLP)
Each plays a pivotal role in safeguarding public health and maintaining the integrity of pharmaceutical products.
The critical impact of GxP compliance on organizational success cannot be overstated. Adherence to these standards not only mitigates risks but also enhances market credibility and fosters trust among stakeholders. For instance, industry leaders like AVS Life Sciences and White Raven demonstrate that a commitment to GxP principles can lead to improved operational capabilities and successful regulatory outcomes. As the life sciences landscape evolves, staying informed about GxP variations and regulatory expectations will be paramount for compliance officers.
Ultimately, prioritizing GxP compliance transcends mere regulatory necessity; it serves as a strategic advantage that significantly influences patient safety and satisfaction. The call to action for organizations is clear: embracing GxP standards will not only ensure compliance but also pave the way for innovation and excellence in the highly regulated life sciences industry. As this sector continues to grow, understanding and implementing GxP principles will be crucial for maintaining a competitive edge and ensuring the highest quality of products for consumers.
Frequently Asked Questions
What does GxP stand for?
GxP stands for "Good Practice," representing a comprehensive set of regulations and guidelines that govern the quality and integrity of products within the life sciences sector.
What are the main components of GxP?
The main components of GxP include Good Manufacturing Practices (GMP), Good Clinical Practices (GCP), and Good Laboratory Practices (GLP).
Why are GxP guidelines important in the pharmaceutical industry?
GxP guidelines are important because they ensure that products are consistently manufactured and managed according to established quality standards, thereby safeguarding public health and ensuring the efficacy of pharmaceuticals, biotechnology products, and medical devices.
How do GxP guidelines impact product quality?
GxP guidelines serve as the foundation for regulatory compliance, compelling organizations to adhere to stringent quality assurance protocols, including meticulous documentation practices and effective internal auditing methods.
Can you provide an example of GxP implementation?
An example of GxP implementation is AVS Life Sciences' collaboration with a leading biotechnology firm, where they upgraded the client's manufacturing environment from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, ensuring compliance with regulatory standards and enhancing quality assurance processes.
What is the future significance of GxP in life sciences regulations?
By 2025, the significance of GxP in life sciences regulations is expected to rise, necessitating that companies adopt these guidelines effectively to maintain their competitive edge and ensure patient safety.
What is required for successful GxP implementation in pharmaceutical companies?
Successful GxP implementation requires a steadfast commitment to continuous improvement and adherence to best practices, which helps elevate quality and cultivate trust with stakeholders.
