What Does FDA Cleared Mean? Key Insights and Context

Overview
The term 'FDA cleared' delineates the process through which the FDA evaluates medical devices for safety and effectiveness, primarily based on their substantial equivalence to existing products. This process primarily pertains to moderate-risk Class II devices. Notably, this clearance process is less stringent than FDA approval, resulting in approximately 90% of 510(k) submissions being cleared annually. This facilitates expedited market access for manufacturers while simultaneously ensuring consumer safety.
Introduction
Understanding the nuances of medical product regulation is crucial in an industry where safety and efficacy are paramount. The term "FDA cleared" holds significant weight, particularly for Class II devices that are deemed moderate-risk and allowed to enter the market based on their substantial equivalence to existing products.
This article delves into the implications of FDA clearance, offering insights into the benefits of this designation while addressing the complexities of navigating the regulatory landscape. What challenges do manufacturers face in achieving this critical approval, and how does it impact their market strategies?
As we explore these questions, we will uncover the vital compliance solutions that can enhance market strategies and ensure successful navigation of regulatory hurdles.
Define FDA Cleared: Understanding the Term
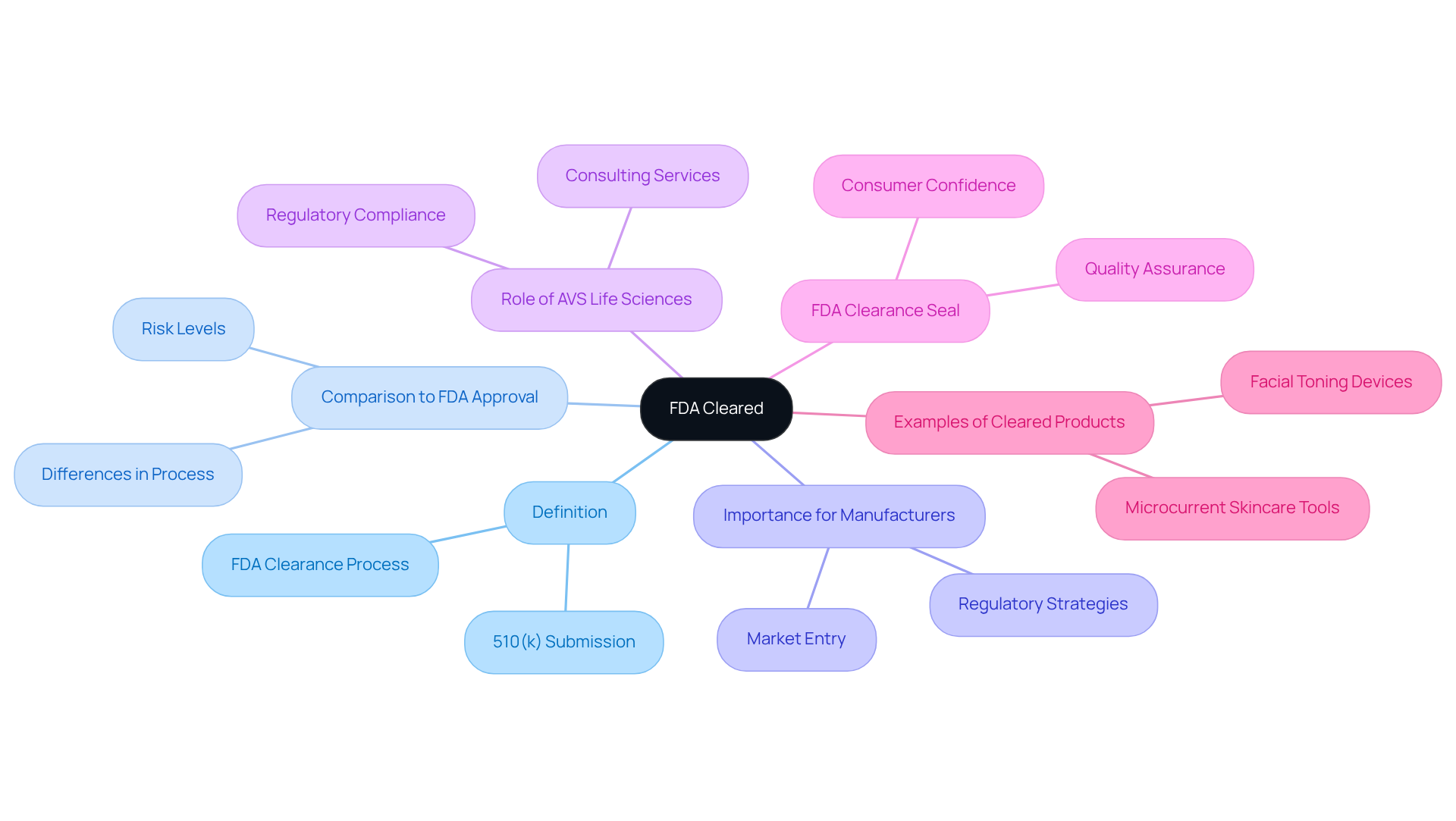
The term 'what does fda cleared mean' refers to the method by which the U.S. Food and Drug Administration (FDA) assesses and approves medical products for sale based on their significant similarity to existing items. This designation primarily applies to Class II items, regarded as moderate-risk.
In contrast to FDA approval, which entails a more stringent procedure necessitating comprehensive clinical trials, the phrase 'what does FDA cleared mean' indicates that the device is considered safe and effective for its intended application based on comparisons to a similar device already available. Approximately 90% of 510(k) submissions obtain FDA approval each year, highlighting the efficiency of this pathway.
Understanding is crucial for manufacturers and healthcare providers, as it directly influences regulatory strategies and market entry. AVS Life Sciences plays a vital role in assisting clients through the FDA approval process, providing expert guidance in quality management and regulatory compliance.
The FDA clearance seal is not just a label; it assures quality, safety, and efficacy, reinforcing consumer confidence in the products. Examples of FDA-cleared products, such as microcurrent skincare tools, demonstrate significant market impact, as they have undergone rigorous testing and evaluation, ensuring they deliver promised results.
This clarity in terminology is essential for avoiding regulatory pitfalls and maintaining trust in the medical equipment industry.
Context of FDA Clearance in Medical Regulation
In the context of the regulatory framework governing the medical apparatus sector in the United States, it is important to understand what does fda cleared mean, as FDA approval stands as a pivotal element. The FDA's Center for Devices and Radiological Health (CDRH) administers this , which is essential for ensuring that devices are safe and effective for public use.
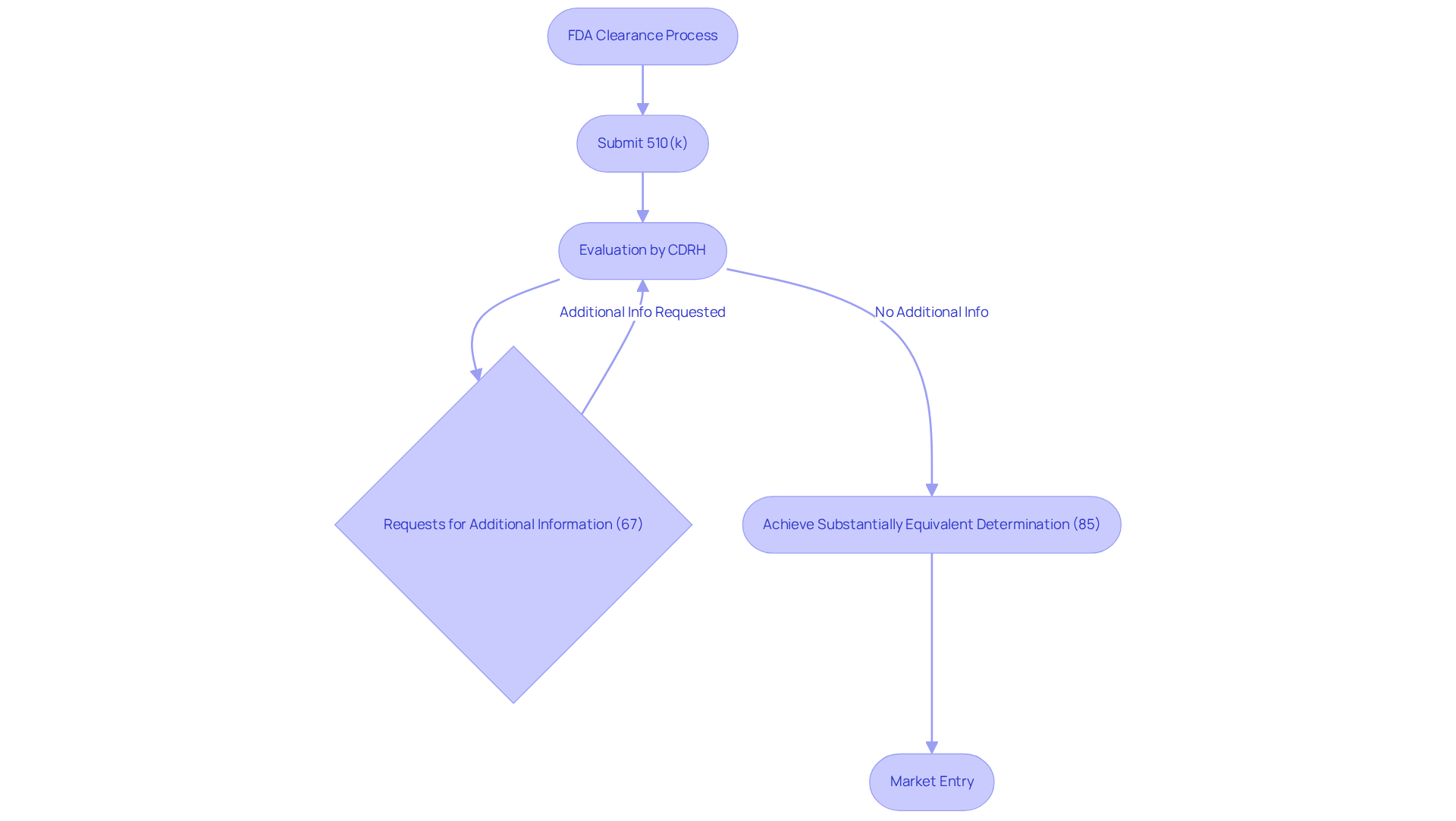
The 510(k) submission method serves as the primary pathway for manufacturers seeking FDA approval, requiring them to demonstrate that their product is substantially equivalent to a legally marketed predicate. In 2023, the FDA received approximately 19,100 submissions and granted a total of 5,807 marketing submissions, marking a slight increase from the previous year. Notably, 85 percent of 510(k) applications achieved a Substantially Equivalent determination, underscoring its role in facilitating timely market entry for innovative products.
However, challenges remain; for instance, 67 percent of submissions prompted requests for additional information during the substantive evaluation, potentially leading to delays and increased costs. Case studies illustrate the necessity of expert regulatory support in navigating these complexities, as many requests for additional information are often avoidable.
The CDRH's focus on enhancing safety and effectiveness through rigorous evaluation methods is crucial for balancing innovation with patient welfare, which raises the question of what does fda cleared mean in the context of medical product regulation.
AVS Life Sciences offers comprehensive regulatory and quality solutions tailored for the medical sector, including support with 510(k) submissions, compliance strategies, and quality management systems. By partnering with knowledgeable regulatory advisors, clients can more effectively navigate the intricacies of FDA approval and address potential challenges.
Historical Evolution of FDA Clearance Processes
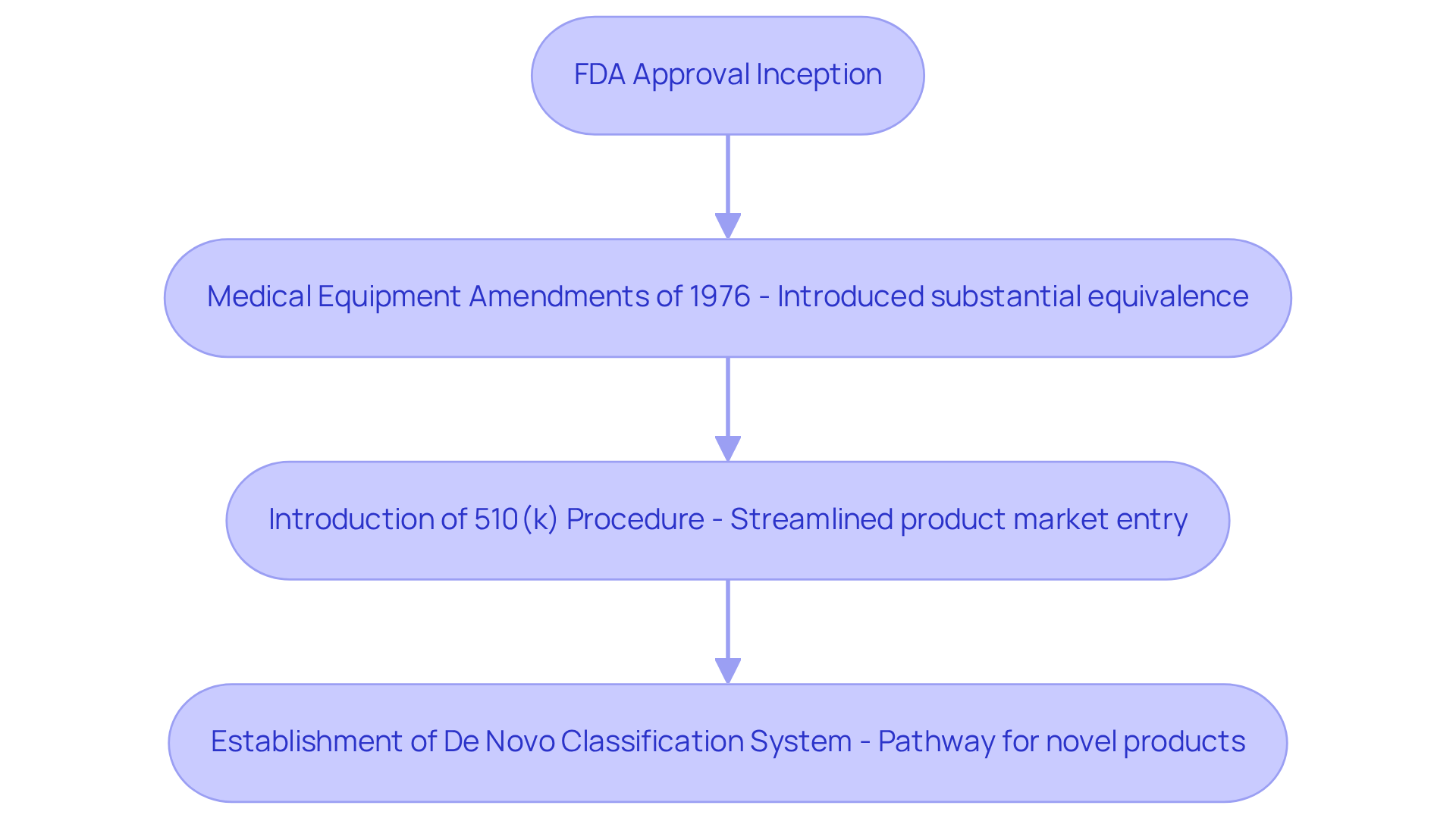
The FDA approval procedure has undergone significant development since its inception, particularly following the Medical Equipment Amendments of 1976, which laid the groundwork for medical apparatus regulation and introduced the concept of substantial equivalence. This pivotal legislation marked a transformative shift in the evaluation of equipment for safety and efficacy. Over the years, the FDA has continually refined its approaches to enhance efficiency and transparency, adapting to technological advancements and responding to industry feedback.
Key milestones include:
- The introduction of the 510(k) procedure, which streamlined the pathway for manufacturers to bring products to market, significantly reducing the time and resources required for approval.
- The establishment of the De Novo classification system, which has provided a pathway for novel products lacking a predicate, further fostering innovation in the sector.
Understanding this historical context is vital for stakeholders in the life sciences industry, especially pharmaceutical compliance officers, as it informs current regulatory practices and shapes expectations for future developments.
A noteworthy case study exemplifying this is ' successful upgrade of a biotechnology GMP facility for a leading San Francisco-based company. This project not only complied with stringent quality assurance standards but also demonstrated regulatory adherence, enabling the client to concentrate on developing targeted antibodies for cancer. Such case studies highlight the essential role of compliance in safeguarding public health and underscore the operational excellence required to navigate FDA regulations.
Key Characteristics of FDA Clearance vs. Approval
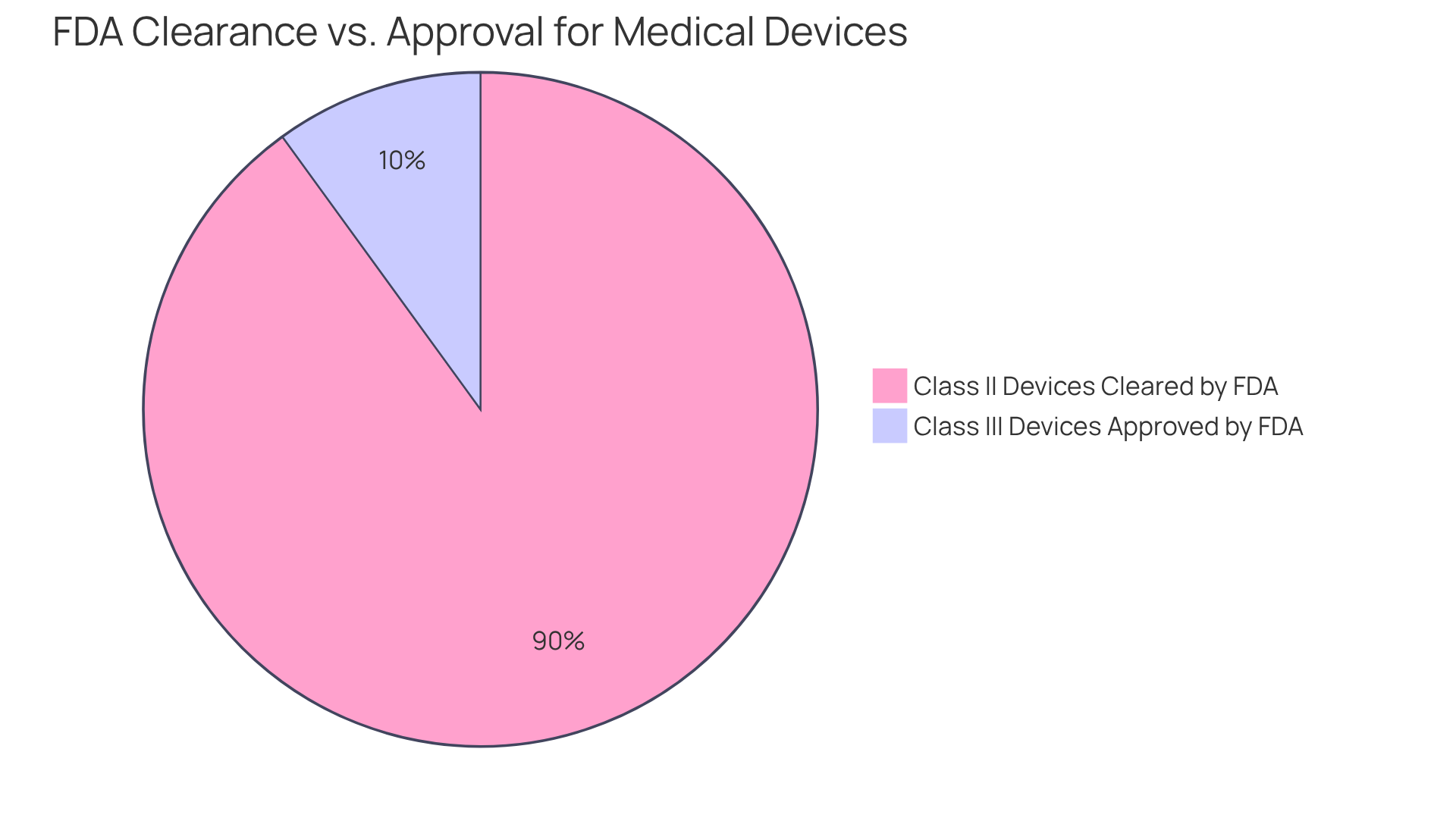
The fundamental differences between FDA authorization and FDA approval are rooted in regulatory requirements and the degree of scrutiny each process entails. FDA approval necessitates demonstrating significant similarity to an existing apparatus, typically requiring less extensive clinical information. In contrast, FDA approval is mandated for Class III products, which pose higher risks and require comprehensive clinical trials to validate safety and efficacy. This distinction holds particular significance for manufacturers, as it directly influences their , timelines, and market entry pathways.
For instance, approximately 90% of Class II devices receive FDA clearance through the 510(k) process, facilitating quicker market access compared to the more rigorous premarket approval (PMA) process necessary for Class III devices. By comprehending these differences, stakeholders can make informed decisions regarding product development and regulatory compliance, ultimately enhancing their strategic positioning in the competitive landscape of the life sciences industry.
Conclusion
Understanding the meaning of 'FDA cleared' is essential for anyone involved in the medical device industry. This designation signifies that a product has been evaluated for safety and effectiveness based on its substantial equivalence to existing devices. Unlike the more rigorous FDA approval process, which demands extensive clinical trials, the FDA clearance pathway allows for a more streamlined entry into the market, particularly for Class II devices.
The article delves into the intricacies of the FDA clearance process, highlighting the importance of the 510(k) submission method and its role in facilitating timely market access. It also underscores the historical evolution of FDA regulations and the critical distinctions between FDA clearance and approval. By recognizing these nuances, stakeholders can navigate the regulatory landscape more effectively and make informed decisions that enhance their strategic positioning in the life sciences sector.
As the medical device industry continues to evolve, the significance of FDA clearance cannot be overstated. It ensures that products meet safety and efficacy standards while fostering innovation and consumer trust. Engaging with knowledgeable regulatory advisors is crucial for manufacturers to streamline their compliance processes and address potential challenges. This collaboration ultimately contributes to improved outcomes in healthcare. Embracing the complexities of FDA clearance is vital for advancing public health and maintaining confidence in medical technologies.
Frequently Asked Questions
What does "FDA cleared" mean?
"FDA cleared" refers to the method by which the U.S. Food and Drug Administration (FDA) assesses and approves medical products for sale based on their significant similarity to existing items, primarily applying to Class II items, which are considered moderate-risk.
How does FDA clearance differ from FDA approval?
FDA clearance indicates that a device is considered safe and effective for its intended use based on comparisons to a similar device already on the market, whereas FDA approval involves a more stringent process requiring comprehensive clinical trials.
What percentage of 510(k) submissions receive FDA clearance?
Approximately 90% of 510(k) submissions obtain FDA clearance each year, highlighting the efficiency of this pathway.
Why is understanding FDA clearance important for manufacturers and healthcare providers?
Understanding FDA clearance is crucial for manufacturers and healthcare providers as it directly influences regulatory strategies and market entry.
What role does AVS Life Sciences play in the FDA approval process?
AVS Life Sciences assists clients through the FDA approval process by providing expert guidance in quality management and regulatory compliance.
What does the FDA clearance seal signify?
The FDA clearance seal assures quality, safety, and efficacy, reinforcing consumer confidence in the products.
Can you provide an example of an FDA-cleared product?
An example of an FDA-cleared product is microcurrent skincare tools, which have undergone rigorous testing and evaluation to ensure they deliver promised results.
Why is clarity in terminology regarding FDA clearance important?
Clarity in terminology is essential for avoiding regulatory pitfalls and maintaining trust in the medical equipment industry.
