What CAPA Stands For: Key Insights for Compliance Officers

Overview
CAPA, or Corrective and Preventive Actions, represents a cornerstone in quality management, fundamentally aimed at identifying and resolving issues within product development and manufacturing. This ensures stringent compliance with regulatory standards. The article elucidates the critical role of CAPA by detailing its systematic approach to eradicate root causes of existing problems while simultaneously preventing future issues. This not only enhances product safety and effectiveness but also aligns with essential guidelines, including Good Manufacturing Practices and ISO standards. By implementing CAPA, organizations can significantly improve their compliance posture, leading to more reliable outcomes and greater trust in their products.
Introduction
Understanding the intricacies of quality management is essential for compliance officers, particularly regarding Corrective and Preventive Actions (CAPA). This systematic approach addresses existing problems and proactively prevents future issues, playing a vital role in maintaining compliance with regulatory standards such as GMP and ISO.
However, as organizations navigate the complexities of implementing effective CAPA processes, they may encounter challenges that raise critical questions about the effectiveness and adaptability of these measures in a rapidly evolving regulatory landscape. Addressing these challenges is crucial for ensuring that compliance strategies remain robust and effective.
Define CAPA: Understanding Corrective and Preventive Actions
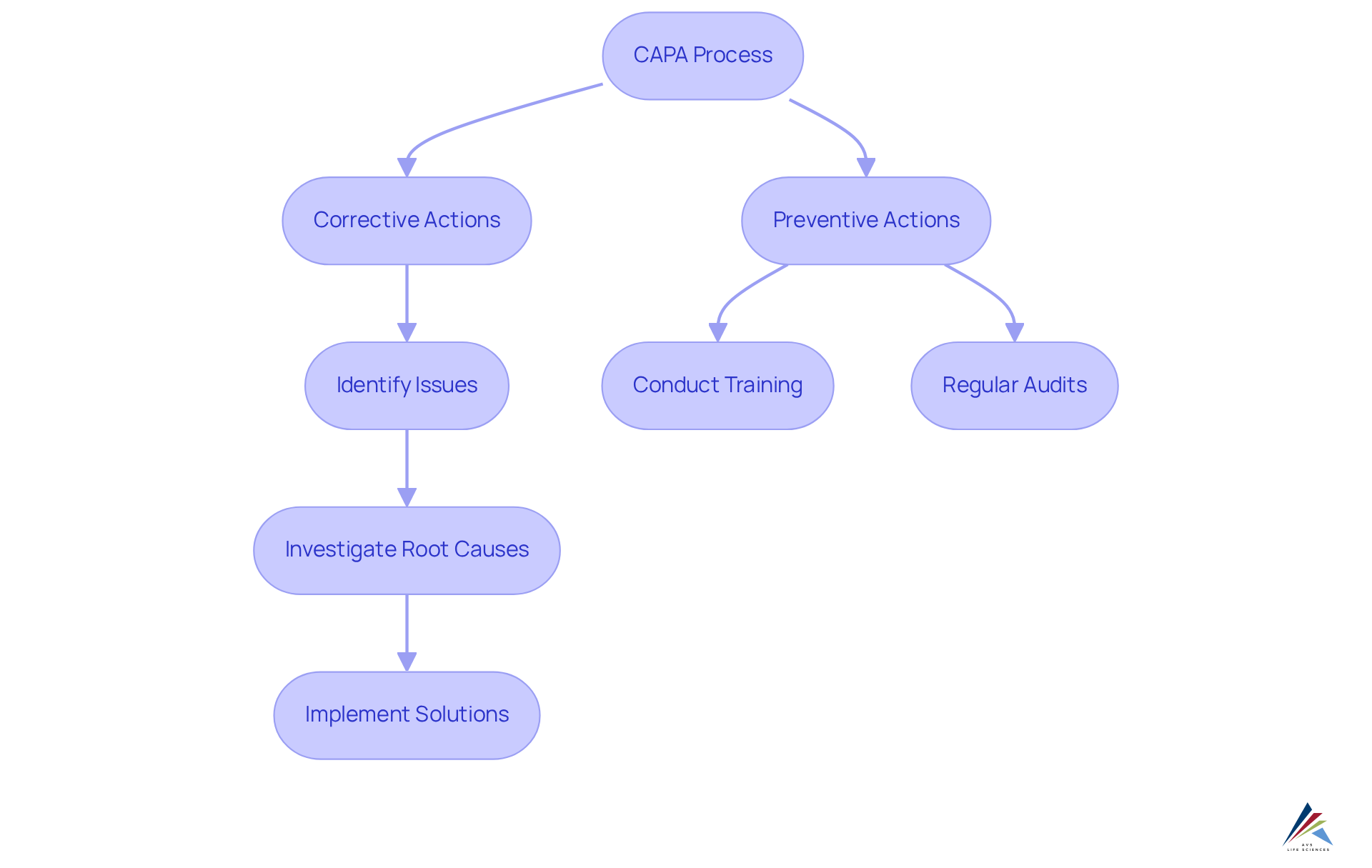
CAPA stands for Corrective and Preventive Actions, and it represents a systematic approach in quality management designed to identify, investigate, and resolve issues that emerge during product development, manufacturing, or service delivery. Corrective actions focus on addressing existing problems by eliminating their root causes, whereas preventive actions aim to avert potential issues from arising in the future. This dual approach is crucial for maintaining compliance with standards such as Good Manufacturing Practices (GMP) and ISO standards, ensuring organizations can deliver safe and effective products.
The importance of corrective and preventive actions in regulatory compliance is paramount. Effective implementation of these processes not only aids organizations in adhering to GMP standards but also aligns with ISO 13485:2016 guidelines, which outline the essential management system for medical device manufacturers. A notable case study from AVS Life Sciences showcased a successful transition from a Biosafety Level 1 to a Level 2 GMP facility for lentivirus production. This example underscores how the application of corrective and preventive actions led to significant improvements in Critical to Quality (CTQ) metrics, thereby enhancing product standards and compliance.
Industry leaders consistently emphasize that CAPA stands for the critical role of corrective and preventive actions in quality management. As one authority noted, 'The advantages and disadvantages of pre-approval and post-approval monitoring systems for medical devices have been fiercely discussed... the process plays a vital role in ensuring that any recognized problems are addressed swiftly and efficiently.' This statement highlights the essential function of corrective and preventive actions in and meeting regulatory compliance.
Corrective actions may include revising manufacturing processes to eliminate defects, while preventive actions could involve conducting regular training sessions to reduce the risk of future errors. By establishing robust corrective and preventive action protocols, organizations not only enhance their compliance with GMP and ISO standards but also foster a culture of continuous improvement, ultimately leading to superior product outcomes and increased customer satisfaction. AVS Life Sciences stands as a premier supplier of management and compliance solutions within the life sciences sector, underscoring the importance of corrective and preventive actions in achieving excellence in product safety and effectiveness.
Trace the Origins of CAPA: Historical Context and Development
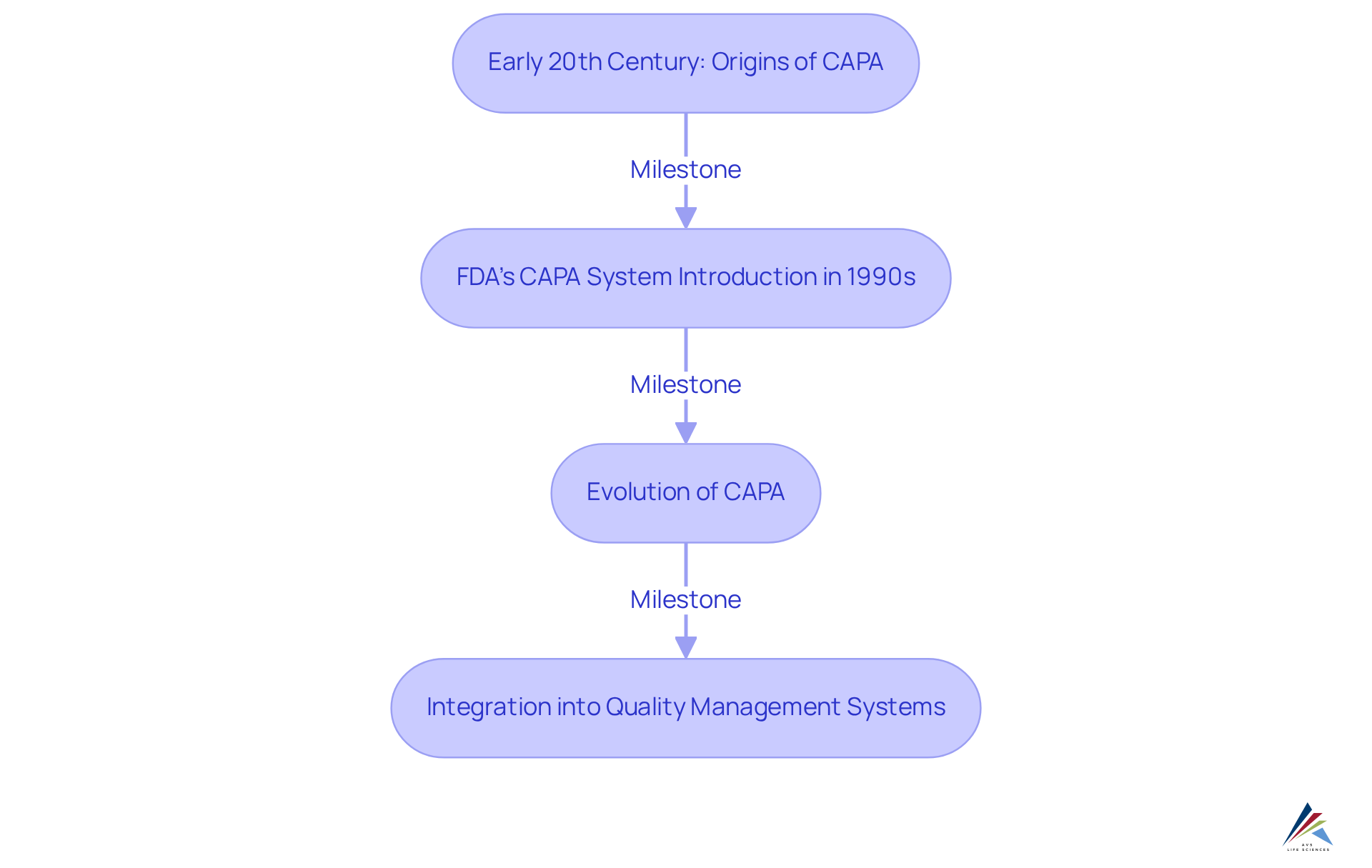
The origins of Corrective and Preventive Measures (CAPA) can be traced back to the early 20th century, a time when what CAPA stands for began to influence standards management in manufacturing processes. The FDA's introduction of a corrective and preventive action system as part of its Quality System Regulation in the 1990s marked a significant milestone in regulatory oversight. This development underscored the critical role of corrective and preventive actions in ensuring product safety and effectiveness, solidifying it as a cornerstone of management systems.
Since its inception, the CAPA program, which CAPA stands for, has evolved significantly, adapting to the complexities of modern regulatory landscapes. The integration of advanced methodologies and technologies has bolstered its effectiveness, enabling organizations to tackle quality issues more proactively. Regulatory specialists emphasize that the evolution of corrective and preventive actions reflects a broader shift towards comprehensive compliance frameworks, particularly within the pharmaceutical and medical device sectors. As Abhishek Raj notes, "The main goal of corrective and preventive measures in any pharmaceutical or medical device sector is to identify the weaknesses, deviations, or failures and to conduct investigations with suitable actions to ensure that such issues do not occur again."
Moreover, the incorporation of what CAPA stands for into a broader Quality Management System (QMS) is essential for ensuring compliance. AVS Life Sciences, a leading provider of management and compliance solutions, offers a range of services including validation solutions, compliance training, and comprehensive audits. Their expertise empowers organizations to navigate compliance challenges effectively while maintaining high standards of quality and adherence. With over 2,500 resources available through The FDA Group, organizations are well-equipped to implement that align with regulatory standards.
Examine Key Components of CAPA: Processes and Methodologies
The essential elements of a corrective and preventive system are critical for effective compliance management. This system encompasses the identification of problems, thorough examination of root causes, execution of remedial measures, and . The process typically follows these structured steps:
- Identify and document the problem
- Evaluate the severity and impact
- Investigate the root cause
- Develop and implement corrective actions
- Verify the effectiveness of the actions taken
- Document the entire process for compliance purposes
Methodologies such as the 5 Whys and Fishbone Diagram are commonly employed to facilitate root cause analysis. These tools ensure that organizations can address not only the symptoms but also the underlying issues, ultimately fostering a culture of continuous improvement and compliance excellence.

Highlight the Importance of CAPA in Regulatory Compliance and Quality Assurance
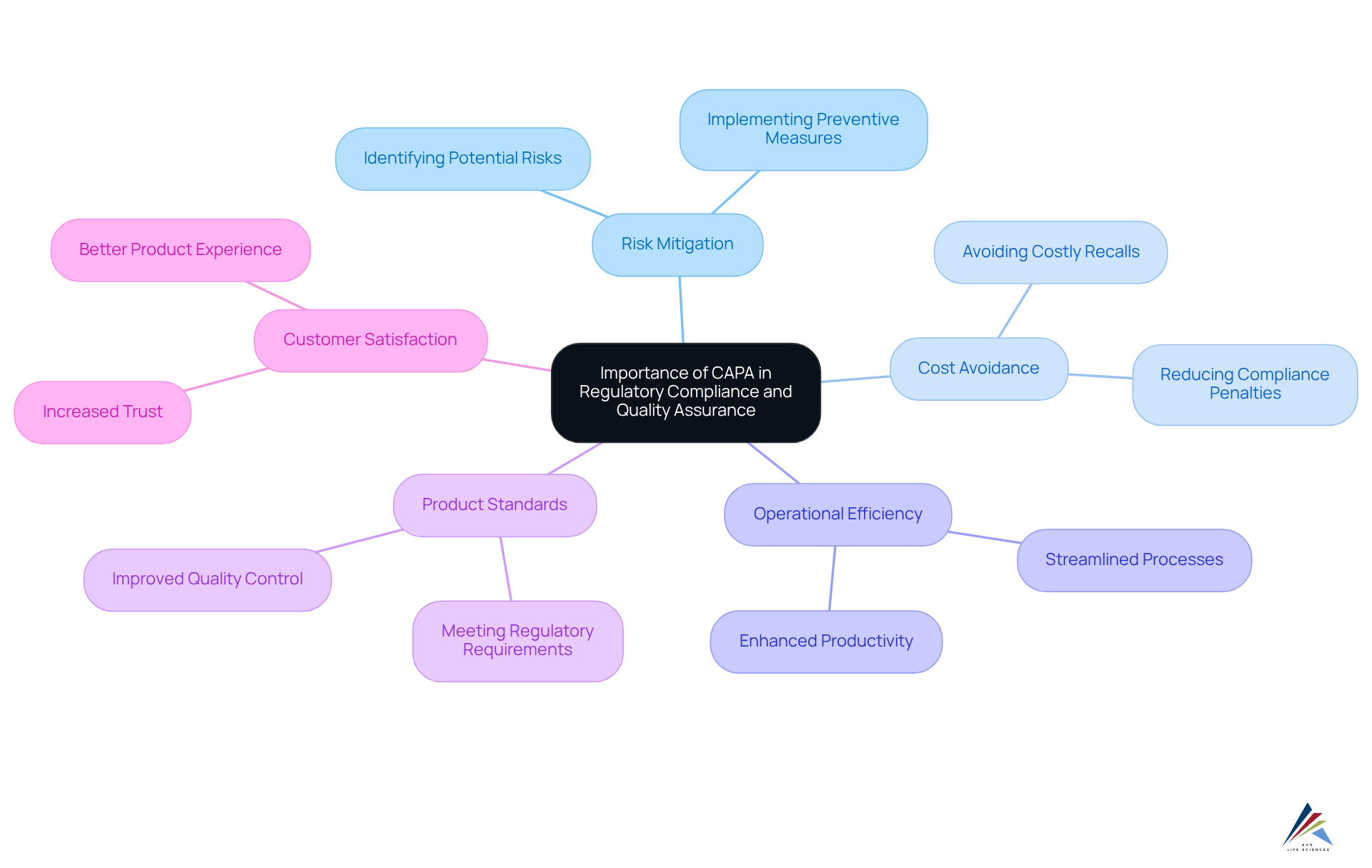
The significance of is paramount, serving as a proactive measure to identify and mitigate risks associated with product standards and compliance. A well-executed corrective and preventive action system, where CAPA stands for such measures, not only helps organizations avoid costly recalls and compliance penalties but also protects their reputation.
Organizations that effectively manage their CAPA processes, which CAPA stands for Corrective and Preventive Action, often report enhanced operational efficiency, improved product standards, and increased customer satisfaction. Moreover, regulatory bodies, including the FDA and ISO, mandate that organizations implement robust CAPA systems, as CAPA stands for the necessity for compliance officers to prioritize these actions within their quality management strategies.
By doing so, they not only ensure compliance but also foster a culture of continuous improvement and accountability.
Conclusion
Understanding the significance of Corrective and Preventive Actions (CAPA) is essential for compliance officers and organizations striving for excellence in quality management. CAPA serves as a structured framework that not only addresses existing issues but also proactively prevents future challenges, thereby ensuring adherence to regulatory standards such as GMP and ISO. By integrating CAPA into their operations, organizations can enhance product safety and effectiveness while fostering a culture of continuous improvement.
Key insights from the article highlight the dual focus of CAPA on both corrective measures—such as revising manufacturing processes—and preventive strategies, like regular training sessions. The historical evolution of CAPA underscores its importance in regulatory compliance, particularly in the pharmaceutical and medical device sectors. Case studies, such as the successful transition at AVS Life Sciences, illustrate the tangible benefits of implementing robust CAPA processes, including improved operational efficiency and heightened customer satisfaction.
Ultimately, the implementation of CAPA is not merely a regulatory requirement but a vital component of a comprehensive quality management system. Organizations are encouraged to prioritize CAPA processes to not only meet compliance standards but also to cultivate a proactive approach to quality assurance. Embracing CAPA can lead to significant improvements in product integrity and organizational reputation, making it an indispensable element in the pursuit of quality excellence.
Frequently Asked Questions
What does CAPA stand for?
CAPA stands for Corrective and Preventive Actions.
What is the purpose of CAPA in quality management?
CAPA represents a systematic approach designed to identify, investigate, and resolve issues that arise during product development, manufacturing, or service delivery.
What is the difference between corrective actions and preventive actions?
Corrective actions focus on addressing existing problems by eliminating their root causes, while preventive actions aim to prevent potential issues from arising in the future.
Why is CAPA important for regulatory compliance?
Effective implementation of CAPA processes aids organizations in adhering to Good Manufacturing Practices (GMP) and aligns with ISO standards, ensuring the delivery of safe and effective products.
Can you provide an example of CAPA in action?
A case study from AVS Life Sciences demonstrated a successful transition from a Biosafety Level 1 to a Level 2 GMP facility for lentivirus production, showing how CAPA led to significant improvements in Critical to Quality (CTQ) metrics.
How do industry leaders view the role of CAPA?
Industry leaders emphasize the critical role of CAPA in quality management, noting its importance in addressing recognized problems swiftly and efficiently to safeguard product integrity and meet regulatory compliance.
What are some examples of corrective and preventive actions?
Corrective actions may include revising manufacturing processes to eliminate defects, while preventive actions could involve conducting regular training sessions to reduce the risk of future errors.
How does establishing CAPA protocols benefit organizations?
Establishing robust CAPA protocols enhances compliance with GMP and ISO standards, fosters a culture of continuous improvement, and ultimately leads to superior product outcomes and increased customer satisfaction.
