Understanding Validation 4.0: The Role of Digital Validation Tools (DVTS)

Overview
Digital Validation Tools (DVTS) are essential in addressing compliance challenges within the pharmaceutical industry, as they automate processes that adhere to regulatory standards like Good Manufacturing Practices (GMP) and ISO standards. By enhancing operational efficiency and reducing human error, these tools support data integrity.
Ultimately, they empower organizations to maintain compliance with stringent regulations while adapting to the evolving landscape of regulatory requirements. As the industry continues to face increasing scrutiny, the adoption of DVTS is not just beneficial; it is imperative for organizations aiming to thrive in a competitive environment.
Introduction
Digital Validation Tools (DVTS) are revolutionizing the pharmaceutical industry. They ensure compliance with stringent regulatory standards while enhancing operational efficiency. As the sector adapts to an increasingly complex landscape, the integration of advanced technologies—such as AI and machine learning—within these tools promises to streamline processes and minimize human error.
However, as organizations embrace this digital transformation, a pressing question arises: how can they effectively leverage these innovations? The goal is not only to meet compliance requirements but also to drive proactive change in their quality management practices.
Define Digital Validation Tools (DVTS) and Their Importance in Pharmaceutical Compliance
Digital Verification Tools are specialized software applications that streamline and automate assessment procedures within the pharmaceutical sector. These tools are indispensable for ensuring that systems and processes comply with regulatory standards, including:
- Good Manufacturing Practices (GMP)
- ISO standards
- Quality System Regulations (QSR)
Their importance is underscored by their capacity to enhance operational efficiency, mitigate human error, and uphold data integrity throughout the verification process. By automating documentation and adherence checks—such as following Standard Operating Procedures (SOPs) and Great Documentation Practices—these systems significantly diminish the risk of non-adherence, which can result in substantial financial penalties and damage to a company's reputation.
Notably, nearly 50% of surveyed experts recognize that data analytics and predictive modeling are pivotal for the future of evaluation, highlighting the transformative potential of validation 4.0 / digital validation tools (dvts) in fostering a proactive regulatory culture. Furthermore, the integration of advanced technologies like AI and machine learning into digital video transport systems is expected to revolutionize assessment methods, with 57% of participants believing these technologies will be essential for evaluation. This evolution ensures that pharmaceutical companies can swiftly adapt to the changing regulatory landscape while maintaining high standards of quality and safety.
Additionally, AVS Life Sciences emphasizes the critical nature of robust quality management practices, including adherence to GXP and FDA regulations, which enhance regulatory alignment by providing greater transparency and traceability—essential in today's complex regulatory environment. As the industry transitions towards more agile and adaptable validation methods, with 46% of respondents anticipating such shifts, the role of validation 4.0 / digital validation tools (dvts) becomes increasingly essential.

Contextualize DVTS Within Regulatory Frameworks and Compliance Standards
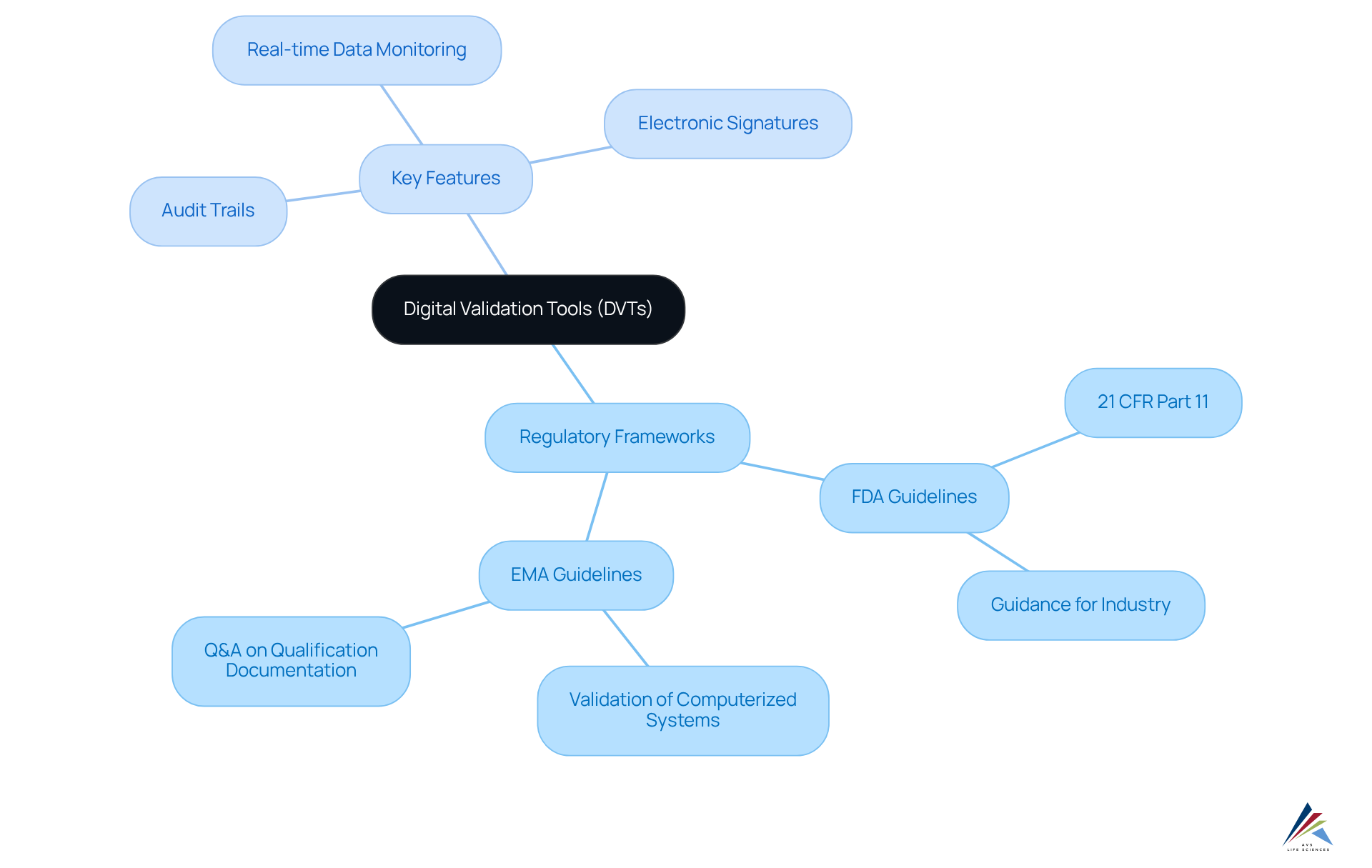
Validation 4.0 / digital validation tools (DVTs) operate within a stringent regulatory framework shaped by guidelines from the FDA and EMA. These regulations mandate pharmaceutical firms to confirm their methods and systems to guarantee adherence to established standards, including Standard Operating Procedures (SOPs). DVTs support this adherence by incorporating essential features such as:
- Audit trails
- Electronic signatures
- Real-time data monitoring
For instance, the FDA's 21 CFR Part 11 specifies the criteria for electronic records and signatures, which DVTs are meticulously designed to meet. This alignment ensures that digital processes maintain the same rigor and reliability as traditional paper-based methods. Moreover, the European Medicines Agency has highlighted the significance of validating computerized systems, thus strengthening the need for validation 4.0 / digital validation tools (DVTs) to ensure adherence to both FDA and EMA standards. By utilizing validation 4.0 / digital validation tools (DVTs), organizations can improve their operational efficiency while adhering to essential regulatory standards. AVS Life Sciences stands out as a leading provider of quality management and regulatory compliance solutions, offering tailored DVT features that help clients navigate these complexities effectively.
Trace the Evolution of Digital Validation Tools in the Pharmaceutical Industry
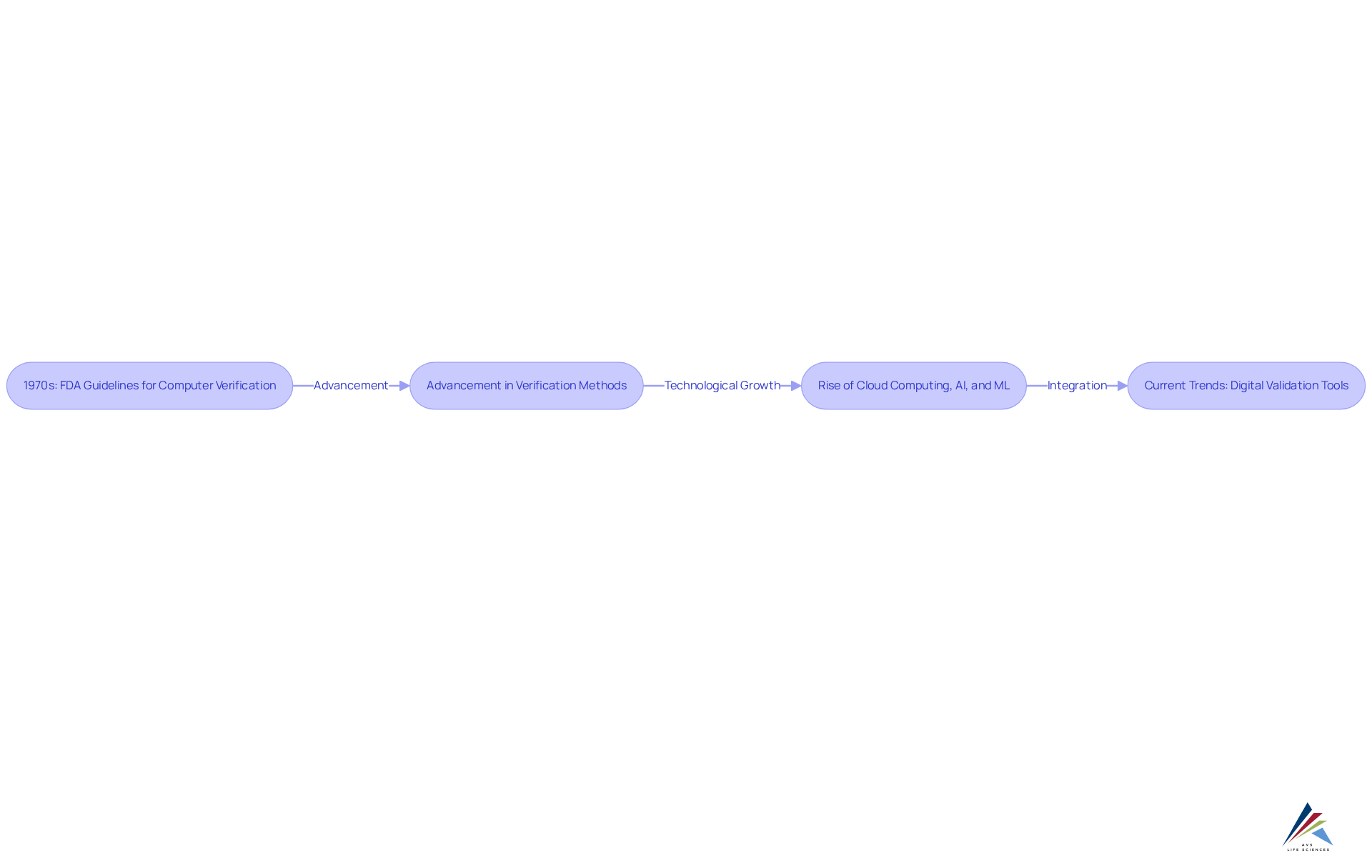
The development of validation 4.0 / digital validation tools (dvts) in the pharmaceutical sector began in the 1970s, marked by the FDA's introduction of guidelines for computer system verification. These guidelines aimed to ensure the reliability of computerized systems. As technology advanced, the complexity of verification methods increased, leading to the creation of more sophisticated tools.
The rise of cloud computing, artificial intelligence (AI), and machine learning (ML) has significantly transformed digital video transport systems, enabling real-time data analysis and enhancing decision-making capabilities. Currently, digital video transport systems play a pivotal role in the verification process, signifying a major shift towards automation and digitalization.
This transformation is underscored by a notable increase in adoption rates; the usage of validation 4.0 / digital validation tools (dvts) has surged dramatically in recent years, reflecting a strong trend towards the integration of these advanced tools within the industry. The incorporation of cloud computing and AI not only enhances the effectiveness of assessment methods but also supports compliance with evolving regulatory requirements, establishing these technologies as fundamental components of modern pharmaceutical operations.
Identify Key Features and Benefits of Digital Validation Tools
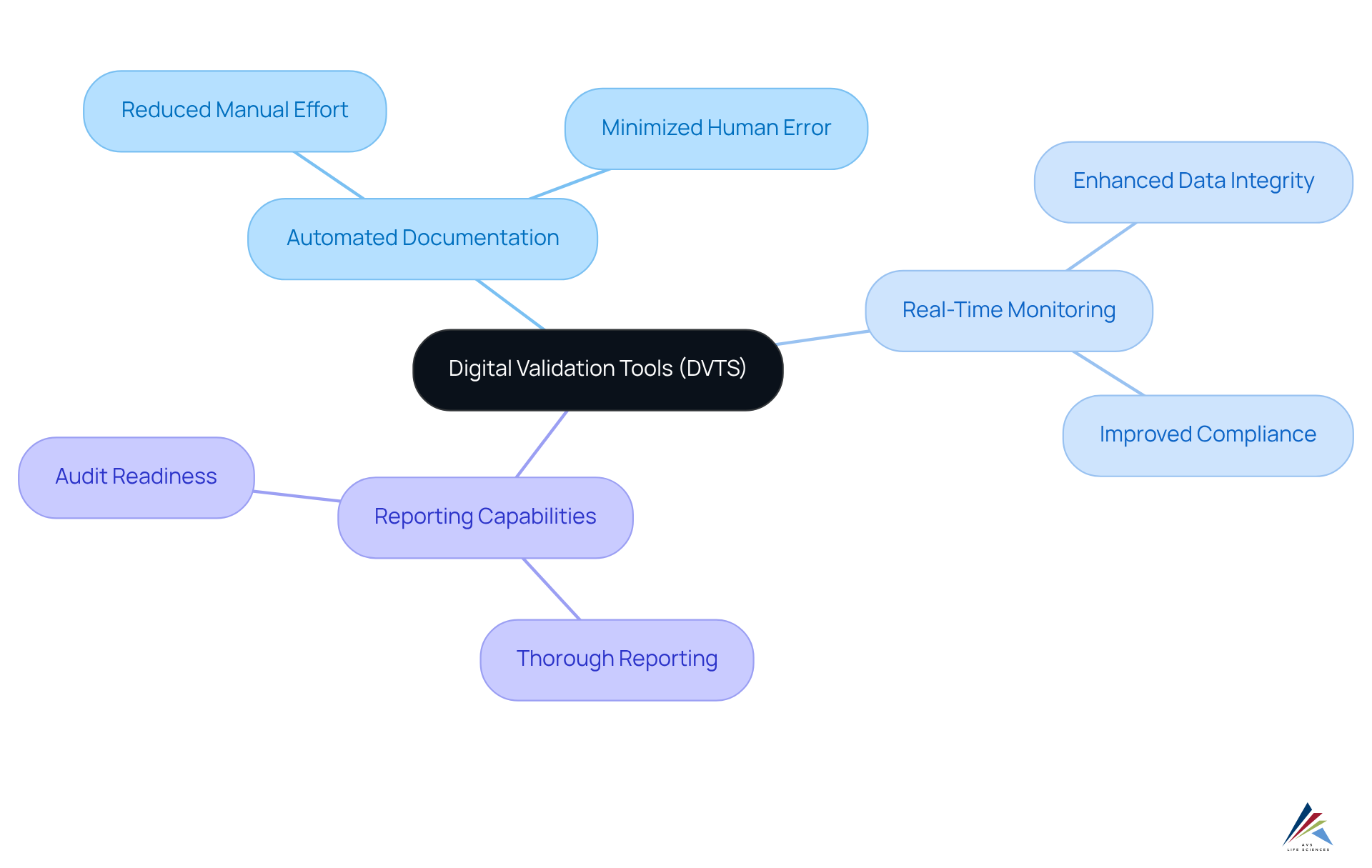
Validation 4.0 / digital validation tools (DVTS) address critical compliance challenges by providing essential features such as:
- Automated documentation
- Real-time monitoring
- Thorough reporting capabilities
These functionalities collectively simplify the assessment process, significantly reducing manual effort and minimizing the risk of human error. As a result, organizations experience enhanced data integrity and improved compliance with regulatory standards. Notably, those who have implemented validation 4.0 / digital validation tools (DVTS) report reductions in verification timelines by up to 50%, leading to substantial cost savings.
For example, automated data validation tools can validate thousands of datasets in mere seconds, enabling companies to maintain detailed electronic records that support audit readiness. This capability not only ensures compliance during inspections but also employs validation 4.0 / digital validation tools (DVTS) to mitigate risks associated with regulatory scrutiny. Furthermore, the integration of validation 4.0 / digital validation tools (DVTS) fosters operational efficiency, allowing pharmaceutical companies to allocate resources towards critical tasks while enhancing their overall quality management strategies.
Conclusion
Digital Validation Tools (DVTS) are indispensable in ensuring compliance within the pharmaceutical industry, facilitating streamlined processes while adhering to stringent regulatory standards. Their integration not only enhances operational efficiency but also significantly mitigates the risk of human error, thereby safeguarding data integrity. As the industry embraces validation 4.0, the significance of these tools becomes increasingly apparent, underscoring their transformative impact on maintaining high standards of quality and safety.
Throughout this article, we have examined key insights into the features and benefits of DVTS. The evolution of these tools, driven by advancements in technology such as AI and machine learning, has led to more sophisticated validation methods. By automating documentation, providing real-time monitoring, and ensuring comprehensive reporting, DVTS empower organizations to reduce verification timelines and enhance compliance with regulatory requirements. This shift towards more agile validation methods reflects a growing recognition of the need for transparency and traceability in today's complex regulatory environment.
As the pharmaceutical landscape continues to evolve, the adoption of digital validation tools will be essential for organizations striving to navigate compliance challenges effectively. The call to action is unequivocal: investing in DVTS not only cultivates a proactive regulatory culture but also positions companies to adapt swiftly to changing regulations, ultimately bolstering their operational resilience and ensuring patient safety. The future of pharmaceutical compliance resides in the strategic implementation of these advanced tools, rendering them crucial for success in an increasingly digital world.
Frequently Asked Questions
What are Digital Validation Tools (DVTS)?
Digital Validation Tools are specialized software applications that streamline and automate assessment procedures within the pharmaceutical sector to ensure compliance with regulatory standards.
Why are DVTS important in the pharmaceutical industry?
DVTS are important because they enhance operational efficiency, mitigate human error, and uphold data integrity throughout the verification process, reducing the risk of non-adherence to regulations.
What regulatory standards do DVTS help comply with?
DVTS help comply with Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR).
How do DVTS improve documentation and adherence checks?
DVTS automate documentation and adherence checks by ensuring compliance with Standard Operating Procedures (SOPs) and Good Documentation Practices, which diminishes the risk of errors.
What are the consequences of non-adherence in the pharmaceutical industry?
Non-adherence can result in substantial financial penalties and damage to a company's reputation.
What role do data analytics and predictive modeling play in the future of evaluation?
Nearly 50% of surveyed experts recognize that data analytics and predictive modeling are pivotal for the future of evaluation, indicating their transformative potential in fostering a proactive regulatory culture.
How are advanced technologies like AI and machine learning expected to impact DVTS?
The integration of AI and machine learning into DVTS is expected to revolutionize assessment methods, with 57% of participants believing these technologies will be essential for evaluation.
What is the significance of robust quality management practices in the pharmaceutical sector?
Robust quality management practices enhance regulatory alignment by providing greater transparency and traceability, which are essential in today's complex regulatory environment.
How is the pharmaceutical industry transitioning in terms of validation methods?
The industry is transitioning towards more agile and adaptable validation methods, with 46% of respondents anticipating such shifts, making the role of DVTS increasingly essential.
