Understanding USP 797 Guidelines: Importance and Key Components

Overview
The USP 797 guidelines represent critical standards for the compounding of sterile preparations. These guidelines are essential in ensuring patient safety and product quality by minimizing contamination risks through strict protocols. Adherence to these guidelines has markedly reduced incidents of contamination and adverse events in healthcare facilities. This underscores the necessity for ongoing compliance and timely updates in response to the evolving challenges within the pharmaceutical industry. As such, it is imperative for all stakeholders to engage actively with these standards to maintain the highest levels of safety and efficacy.
Introduction
The USP 797 guidelines represent a crucial framework established by the United States Pharmacopeia, aimed at ensuring the safety and quality of compounded sterile preparations. Focusing on minimizing contamination risks, these guidelines are essential for healthcare facilities that prepare compounded sterile products, ultimately safeguarding patient health.
However, as the landscape of healthcare continues to evolve, a pressing challenge emerges: how can facilities effectively implement these guidelines amidst varying levels of compliance and regulatory scrutiny?
Exploring the nuances of USP 797 not only illuminates its importance but also raises critical questions about the future of patient safety in sterile compounding practices. The need for robust compliance solutions is evident, prompting a deeper engagement with the strategies that can enhance adherence to these vital standards.
Define USP 797 Guidelines and Their Importance
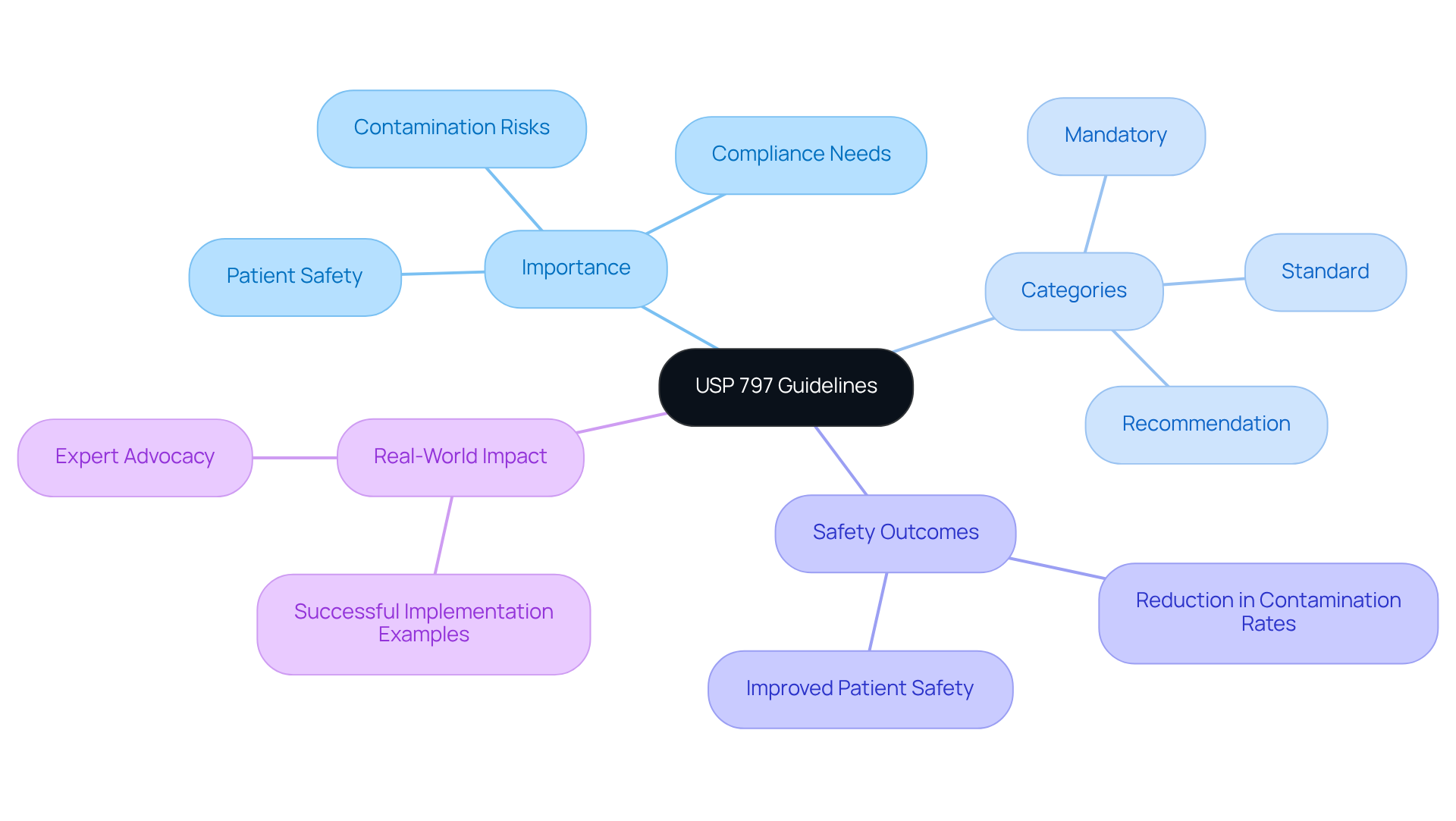
The , established by the United States Pharmacopeia, provide of sterile preparations, ensuring both and product quality. The usp 797 guidelines are essential protocols for healthcare facilities engaged in the preparation of compounded sterile products (CSPs), as they outline the necessary practices and environmental conditions required to minimize contamination risks. The significance of the usp 797 guidelines is highlighted by their protective role against potential harm from microbial contamination, improper labeling, and other compounding errors, thus enabling healthcare providers to deliver safe and effective medications.
Recent updates to the usp 797 guidelines underscore the imperative for stringent , particularly regarding contamination. The protocols are categorized into three tiers: mandatory, standard, and recommendation, aiding healthcare facilities in understanding the varying levels of adherence required. For example, the new standards reveal that contamination of sterile products is the most prevalent error in drug compounding, with substantial patient safety incidents prompting national initiatives to bolster compliance. A study indicated that from 2001 to 2017, over 1,416 negative incidents linked to compounded medications occurred, including 115 fatalities, emphasizing the critical need for adherence to these protocols. Alarmingly, only 30% of states mandate sterile compounding pharmacies to report serious adverse events, highlighting the implications of the regulatory landscape on patient safety.
In October 2011, the Institute for Safe Medication Practices (ISMP) convened a summit, bringing together specialists to formulate protocols for the . This collaborative effort underscores the importance of adhering to these principles in and enhancing overall quality. Real-world examples illustrate the across various healthcare facilities, resulting in improved safety outcomes. Facilities that have adopted these recommendations report reductions in . Expert opinions further reinforce the significance of the usp 797 guidelines, with numerous professionals advocating for their integration into sterile compounding practices. As the healthcare landscape continues to evolve, the unwavering commitment to the usp 797 guidelines remains vital for safeguarding patient health.
Contextualize USP 797 Within Regulatory Frameworks
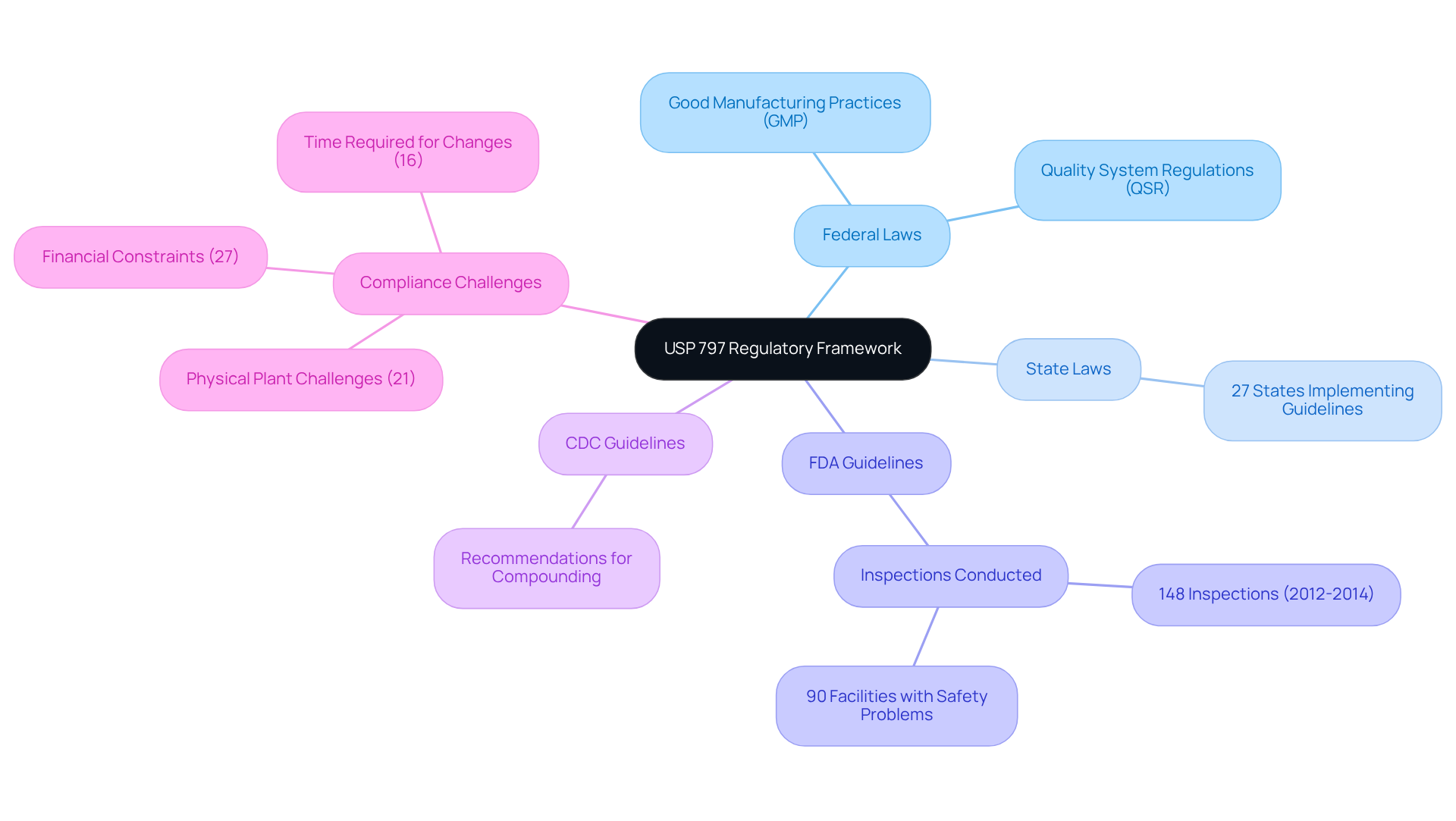
USP 797 operates within a robust regulatory framework that encompasses both federal and state laws, alongside guidelines from the Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC). This framework is meticulously designed to ensure that . By adhering to and , USP 797 significantly enhances the safety of compounded sterile preparations, fostering a culture of adherence throughout healthcare facilities.
, a leading provider of quality management and for the life sciences industry, offers comprehensive and expertise in regulatory compliance. This ensures that pharmaceutical and biotechnology companies meet the necessary standards. Recent statistics reveal that a considerable portion of healthcare facilities are now aligning with , showcasing an increasing dedication to patient safety and regulatory compliance. For instance, 27 states have implemented the , enforcing them to varying extents, which underscores the significance of these guidelines in delivering high-quality healthcare.
' illustrates their successful upgrade of a biotechnology GMP facility, with a focus on quality assurance and regulatory compliance. This example demonstrates the effectiveness of integrating the USP 797 guidelines with FDA and CDC recommendations in promoting safe compounding practices and mitigating risks associated with microbial contamination.
Trace the Evolution of USP 797 Guidelines
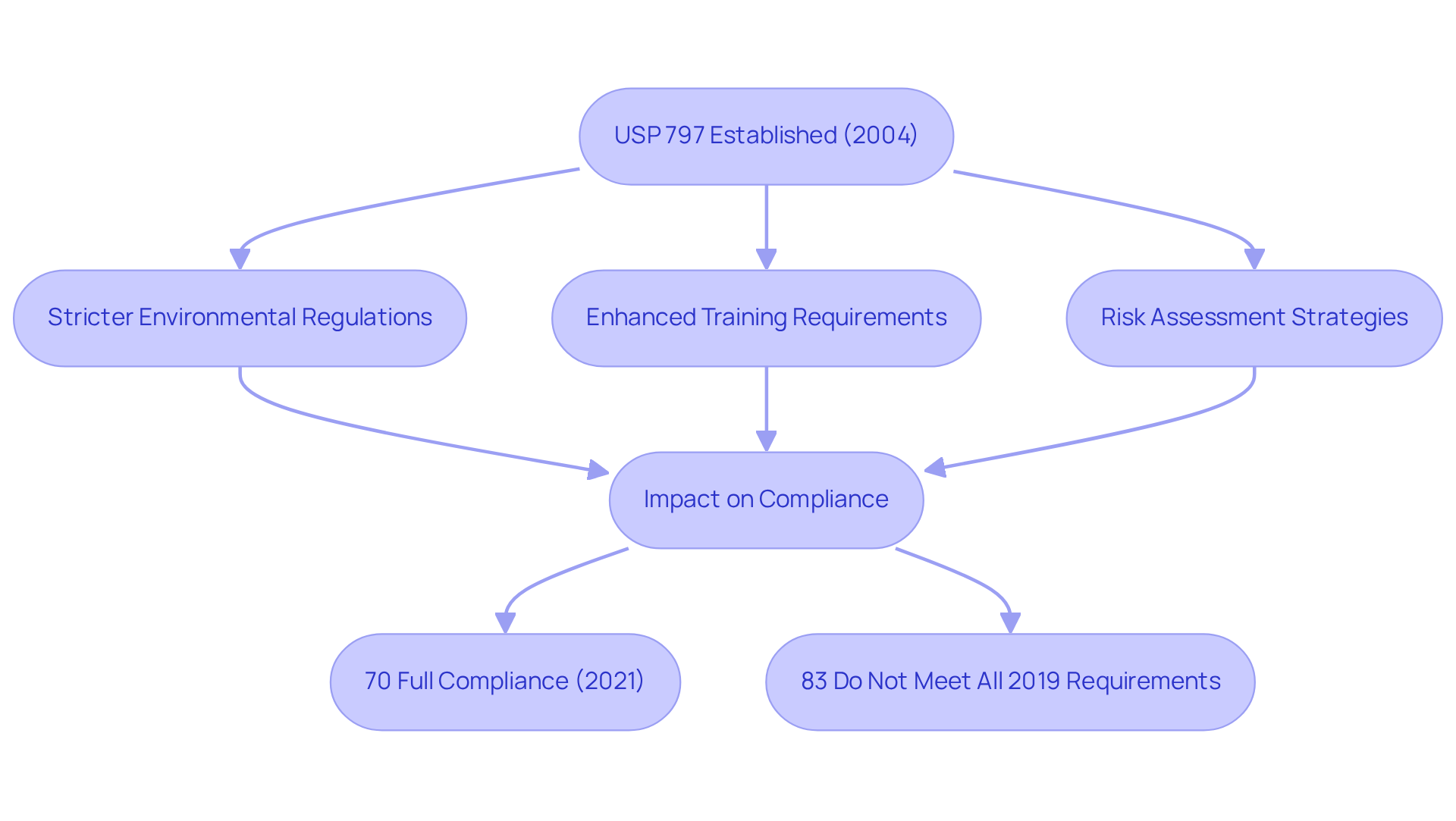
The were first established in 2004 in response to growing concerns regarding the safety of compounded sterile preparations. Since their inception, these protocols have undergone multiple revisions to tackle emerging challenges and integrate advancements in technology. Notable updates have included:
- The implementation of stricter environmental regulations, crucial for minimizing contamination risks.
- Enhanced training requirements for staff to ensure they are equipped to uphold these standards.
- The introduction of risk assessment strategies, empowering facilities to more effectively identify and mitigate potential hazards within sterile compounding environments.
These revisions reflect a steadfast commitment to and quality assurance within the pharmaceutical industry. Prior to the enforcement of the USP 797 guidelines, statistics indicated that incidents of microbial contamination were alarmingly prevalent, highlighting the necessity for these stringent guidelines. Post-revision, adherence rates among hospital pharmacies have seen significant improvement, with a 2021 survey revealing that 70% reported full compliance with the standards. However, it is critical to recognize that 83% of currently compliant pharmacies do not yet fulfill all requirements necessary for complete adherence to the 2019 standard.
This evolution of the USP 797 guidelines exemplifies a proactive stance towards and enhancing the overall efficacy of . A transformative case study from AVS Life Sciences exemplifies this commitment: the company successfully assisted a leading biotechnology firm in upgrading their , ensuring compliance with rigorous . This collaboration not only allowed the client to concentrate on the development of life-saving medicines but also underscored how directly addressed the evolving standards of USP 797, highlighting the paramount importance of comprehensive quality management processes in achieving .
As Brandon Russell, Solutions Marketing Leader for Life Science Solutions, asserts, transcends mere regulatory compliance; it represents an ethical obligation to safeguard patient safety.
Outline Key Components and Requirements of USP 797
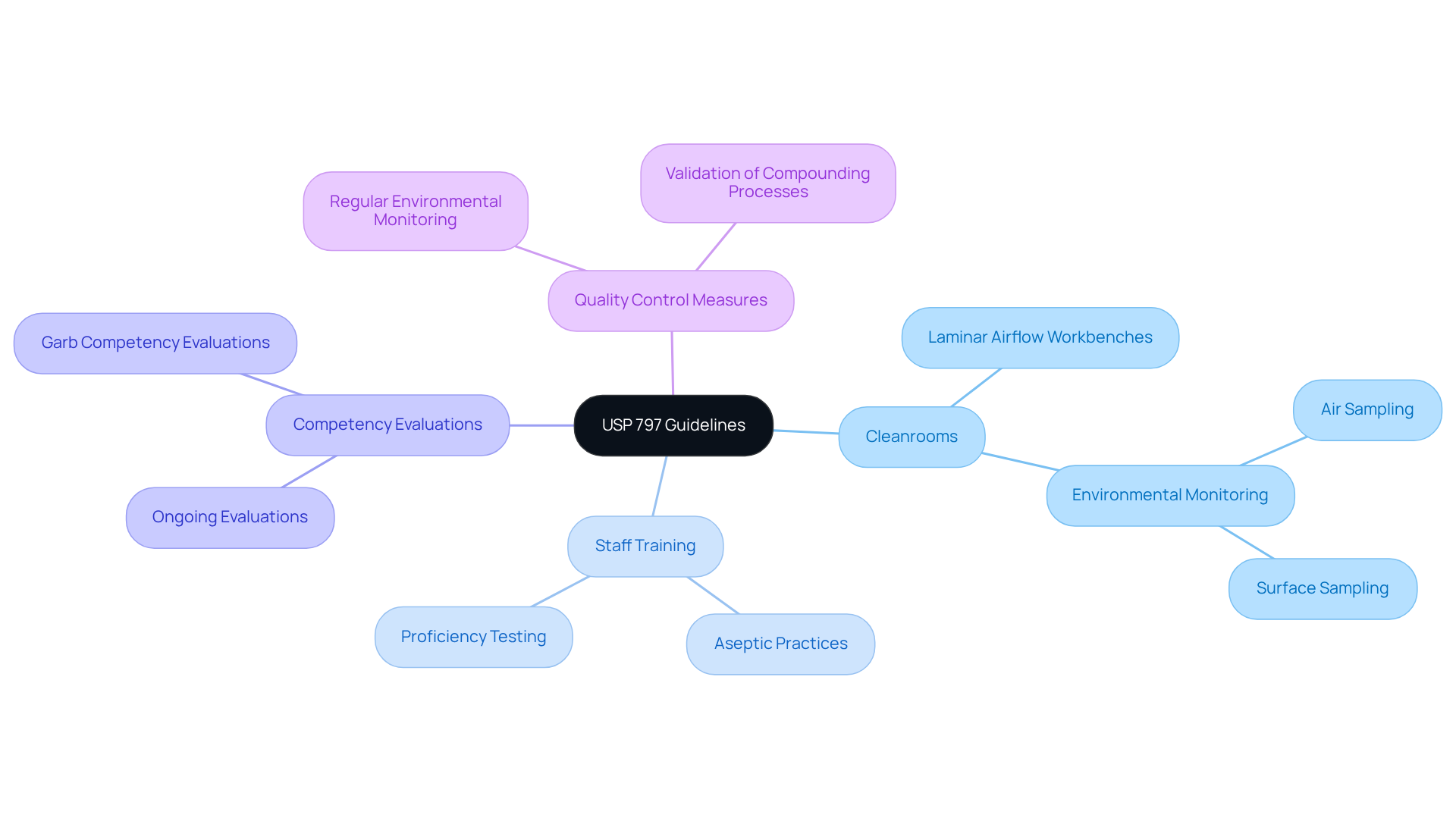
The USP 797 guidelines establish critical components for a , emphasizing the necessity of cleanrooms and laminar airflow workbenches to mitigate contamination risks. A key element of these protocols is the imperative for and the correct use of personal protective equipment (PPE).
As of November 1, 2023, will be mandatory, reflecting a steadfast commitment to patient safety and quality assurance. Furthermore, USP 797 mandates , including regular environmental monitoring and validation of compounding processes. These measures are essential for ensuring that compounded sterile products are not only safe for patient use but also meet the highest quality standards.
AVS Life Sciences offers that ensure compliance with these standards, focusing on GXP and FDA regulations. adherence are vital, emphasizing to ensure personnel are well-prepared to maintain sterile conditions during compounding.
By adhering to these guidelines and leveraging AVS Life Sciences' , healthcare facilities can significantly diminish the risk of microbial contamination, thereby safeguarding patient health.
Conclusion
The USP 797 guidelines are pivotal in ensuring the safety and quality of compounded sterile preparations, underscoring the necessity of rigorous protocols within healthcare environments. By establishing definitive standards for compounding practices, these guidelines act as a safeguard against contamination and compounding errors, ultimately protecting patient health.
Key arguments throughout the article reinforce the importance of adhering to these guidelines, including:
- The implementation of environmental controls
- Staff training
- Continuous compliance assessments
The evolution of USP 797 illustrates a commitment to tackling emerging challenges in the pharmaceutical sector, with updates aimed at bolstering patient safety. Real-world examples highlight the beneficial impact of these guidelines, showcasing enhanced safety outcomes in healthcare facilities that have adopted these standards into their operations.
The importance of the USP 797 guidelines transcends mere compliance; it embodies a shared responsibility to prioritize patient safety in the compounding process. As the healthcare landscape continues to evolve, embracing these guidelines and nurturing a culture of adherence will be essential in mitigating risks associated with compounded sterile products. The dedication to these standards not only improves the quality of care delivered but also reinforces the ethical obligation to shield patients from preventable harm.
Frequently Asked Questions
What are the USP 797 guidelines?
The USP 797 guidelines, established by the United States Pharmacopeia, provide critical standards for the compounding of sterile preparations to ensure patient safety and product quality.
Why are the USP 797 guidelines important?
They are essential for healthcare facilities preparing compounded sterile products (CSPs) as they outline practices and environmental conditions needed to minimize contamination risks, protecting against potential harm from microbial contamination and compounding errors.
What recent updates have been made to the USP 797 guidelines?
Recent updates emphasize stringent environmental controls and comprehensive risk assessments, particularly regarding contamination, and categorize protocols into mandatory, standard, and recommendation tiers.
What is the significance of contamination in drug compounding?
Contamination of sterile products is the most common error in drug compounding, leading to significant patient safety incidents and prompting national initiatives to improve compliance with the USP 797 guidelines.
What statistics highlight the importance of adhering to USP 797 guidelines?
A study indicated that from 2001 to 2017, there were over 1,416 negative incidents linked to compounded medications, including 115 fatalities, underscoring the critical need for adherence to these protocols.
How many states require sterile compounding pharmacies to report serious adverse events?
Only 30% of states mandate sterile compounding pharmacies to report serious adverse events, which has implications for patient safety.
What collaborative efforts have been made to enhance the safety of compounded sterile preparations?
In October 2011, the Institute for Safe Medication Practices (ISMP) convened a summit with specialists to formulate protocols for the safe preparation of compounded sterile preparations.
What are the outcomes of implementing USP 797 guidelines in healthcare facilities?
Facilities that have adopted the USP 797 guidelines report reductions in contamination rates and improved patient safety.
What is the general consensus among experts regarding USP 797 guidelines?
Numerous professionals advocate for the integration of USP 797 guidelines into sterile compounding practices, reinforcing their significance in safeguarding patient health.
