Understanding the Supplement Manufacturing Process in Life Sciences

Overview
The supplement manufacturing process in life sciences encompasses several critical stages:
- Sourcing raw materials
- Formulation
- Production
- Quality assurance
- Packaging
- Distribution
Each stage is meticulously designed to ensure product safety, efficacy, and quality. It is paramount to adhere to regulatory standards, such as Good Manufacturing Practices (GMP) and FDA regulations, as these foster consumer trust and enhance the integrity of dietary supplements in an increasingly competitive market. By prioritizing compliance, manufacturers can not only meet legal requirements but also position themselves as leaders in the industry. This commitment to quality and safety ultimately drives consumer confidence and supports market growth.
Introduction
The supplement manufacturing process stands at the intersection of health and innovation, reflecting a growing consumer demand for dietary enhancements that promote well-being. This intricate journey—from sourcing raw materials to ensuring regulatory compliance—plays a pivotal role in delivering safe and effective products to the market.
However, as the industry evolves, manufacturers face significant compliance challenges. How can they navigate the complexities of quality assurance and regulatory standards while meeting the rising expectations of health-conscious consumers?
This article will explore these challenges, providing detailed solutions and actionable insights for industry professionals.
Defining the Supplement Manufacturing Process
The represents a systematic approach aimed at producing dietary supplements, which encompass vitamins, minerals, herbs, amino acids, and other health-enhancing substances. This comprehensive procedure is part of the supplement manufacturing process and consists of several essential stages:
- Sourcing raw materials
- Creating items
- Production
- Packaging
- Distribution
Each phase of the supplement manufacturing process is crucial in ensuring that the final products meet safety, efficacy, and quality standards, ultimately providing individuals with reliable health benefits. Maintaining high-quality standards throughout the supplement manufacturing process is imperative, as it directly influences product integrity and fosters consumer confidence.
Key features such as Data Integrity Deviations, Investigations, and Standard Operating Procedures (SOPs) are vital components of the supplement manufacturing process, ensuring adherence to GXP and FDA regulations. By understanding and refining the manufacturing method, stakeholders in the life sciences can effectively navigate the complexities of product development and enhance their contributions to the health and wellness industry.
Context and Importance in the Life Sciences Industry
The supplement manufacturing process has garnered substantial attention in recent years, propelled by a notable surge in consumer interest in health and wellness. As preventive health strategies gain traction, dietary additions have emerged as a favored option for enhancing nutritional intake and promoting overall wellness.
The life sciences industry plays a crucial role in this dynamic, ensuring that the supplement manufacturing process is conducted in strict compliance with rigorous regulatory standards. This commitment to compliance not only protects product safety and efficacy but also strengthens consumer trust within the marketplace.
A transformative case study that exemplifies this is AVS Life Sciences' successful upgrade of a biotechnology GMP facility for a prominent San Francisco-based company. This initiative not only bolstered the facility's adherence to regulatory standards but also refined quality assurance methods. AVS Life Sciences executed the upgrade on schedule and within budget, underscoring their dedication to quality management.
This collaboration enabled the client to concentrate on medicine development while AVS ensured that the complied with necessary regulations. Key lessons learned during this project included the identification of anomalies in test results stemming from improperly installed barcode scanner cameras, which underscored the critical need for stringent quality control measures.
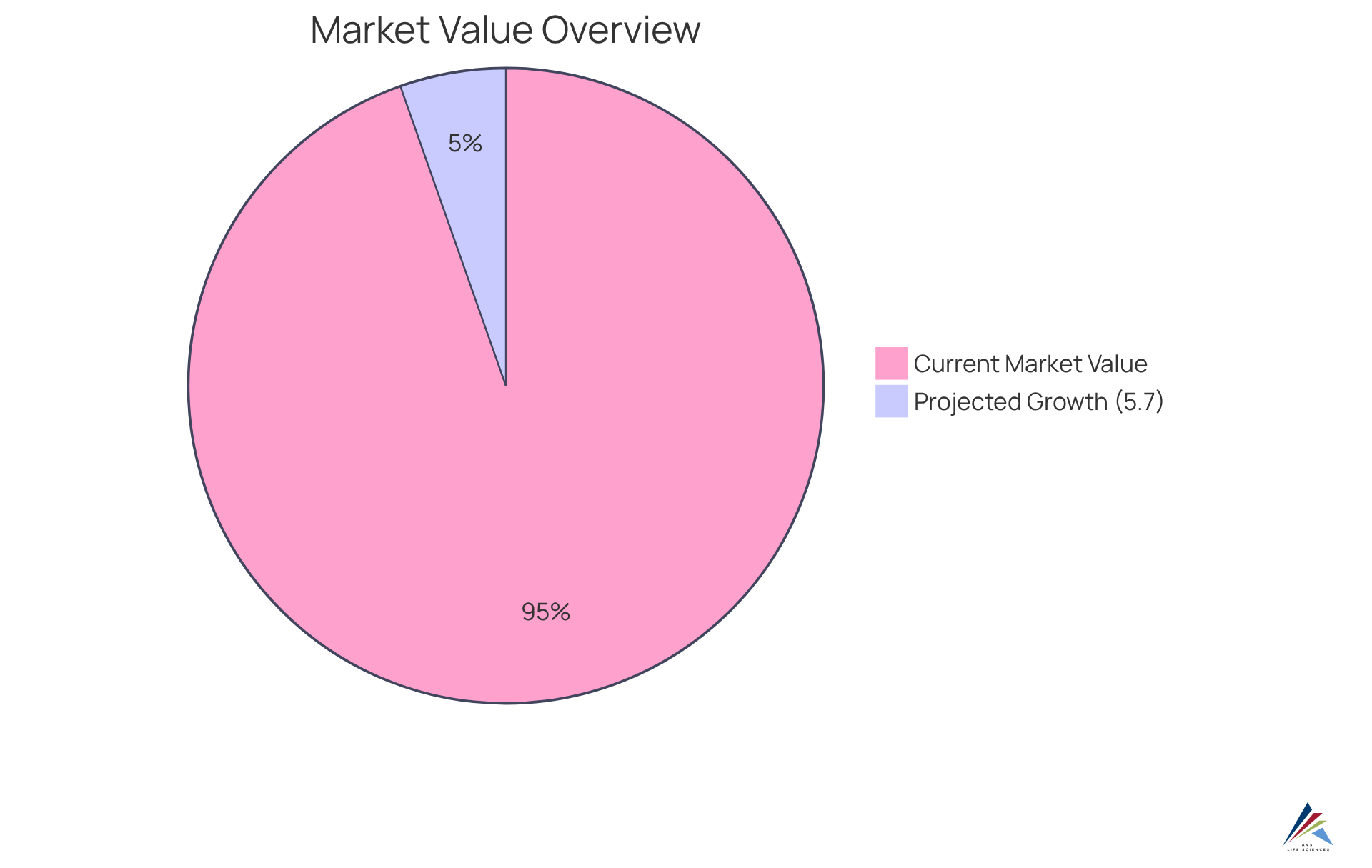
The U.S. dietary health market was valued at $50.91 billion in 2022, with a projected CAGR of 5.7% from 2023 to 2030, reflecting the growing demand for high-quality offerings. Companies that prioritize ethical sourcing and transparent practices are better positioned to meet buyer expectations and navigate the complexities of regulatory frameworks, ultimately fostering a more reliable industry.
Key Stages of the Supplement Manufacturing Process
The supplement manufacturing process encompasses several essential stages that ensure product quality and compliance with industry standards:
- Raw Material Sourcing: This foundational stage focuses on selecting high-quality ingredients that adhere to regulatory and safety standards. It is essential to assess suppliers thoroughly to guarantee they adhere to Good Manufacturing Practices (GMP), which are crucial for preserving quality and safety for users.
- Formulation: During the supplement manufacturing process, manufacturers create specific formulations tailored to deliver the intended health benefits. This process requires in-depth to ensure that the supplements are both effective and safe for consumers.
- Production: The actual supplement manufacturing process takes place in this stage, where raw materials are processed, blended, and formed into capsules or tablets. Strict quality control measures are essential here to prevent contamination and ensure that the items meet safety standards.
- Quality Assurance and Testing: Rigorous testing is conducted during the supplement manufacturing process to verify that the final item meets established specifications for potency, purity, and safety. This encompasses microbiological testing and stability studies, which are vital for confirming the item's efficacy and shelf life.
- Packaging and Labeling: Proper packaging is essential for preserving item integrity and providing buyers with precise information. Labels must adhere to regulatory standards, including detailed ingredient lists and health assertions, to ensure transparency and public trust.
- Distribution: Ultimately, items are allocated to retailers or directly to buyers, with meticulous consideration for storage and transport conditions that maintain their quality. This phase is essential for guaranteeing that the products arrive to users in prime condition, prepared for application.
Regulatory Standards and Quality Assurance in Supplement Manufacturing
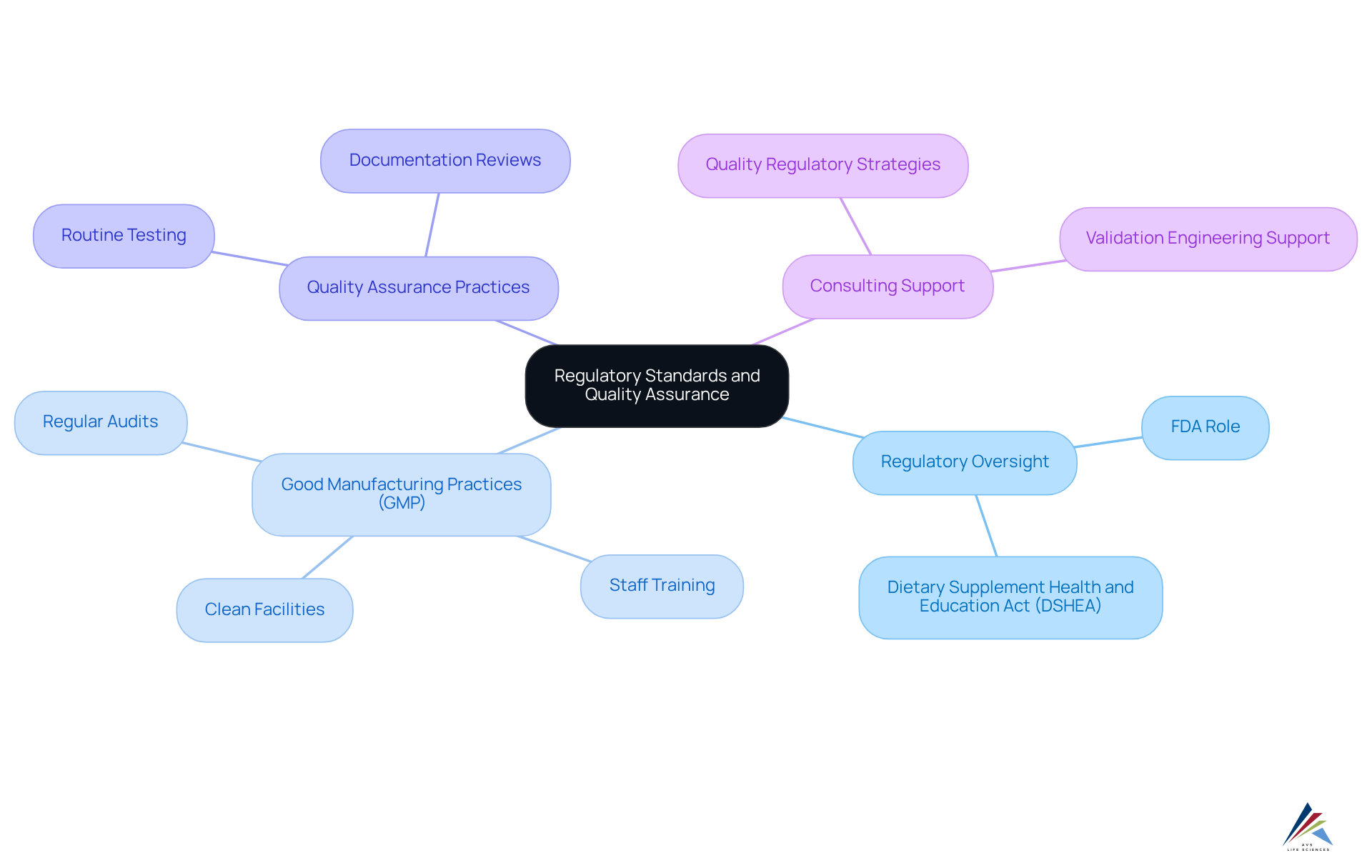
Regulatory standards are pivotal in the supplement manufacturing process, ensuring that products are safe and effective for consumer use. In the United States, the Food and Drug Administration (FDA) oversees dietary supplements under the Dietary Supplement Health and Education Act (DSHEA). This legislation mandates that manufacturers comply with Good Manufacturing Practices (GMP) within the supplement manufacturing process, which includes:
- Maintaining clean facilities
- Properly training staff
- Conducting regular audits
AVS Life Sciences, with a team of over 300 experienced associates, specializes in providing comprehensive consulting services for regulatory compliance and quality management within the life sciences sector. They assist companies in developing effective and offer validation engineering support, ensuring that manufacturers not only meet regulatory requirements but also enhance their credibility and marketability.
Furthermore, manufacturers must ensure that their products are accurately labeled and that any health claims are substantiated by scientific evidence. Quality assurance practices, including routine testing and documentation reviews, are essential for compliance within the supplement manufacturing process and help mitigate risks associated with product recalls or customer complaints. By adhering to these regulatory standards, manufacturers protect consumers and position themselves as reliable players in a competitive industry.
Conclusion
The supplement manufacturing process stands as a cornerstone of the life sciences industry, embodying a systematic approach to producing dietary supplements that adhere to safety and quality standards. By grasping the intricate stages involved—from sourcing raw materials to distribution—stakeholders can ensure that products not only deliver health benefits but also cultivate consumer trust through strict adherence to regulatory requirements.
Key arguments underscore the significance of each phase in the manufacturing process, particularly emphasizing:
- Quality assurance
- Regulatory compliance
- Ethical sourcing
The burgeoning dietary health market highlights the imperative for manufacturers to prioritize transparency and uphold high-quality standards, as evidenced by successful case studies such as AVS Life Sciences' facility upgrades. These elements are essential for navigating the complexities of the industry and addressing the increasing consumer demand for dependable health products.
Ultimately, the supplement manufacturing process plays a pivotal role in advancing health and wellness. As the market continues to expand, it is crucial for manufacturers to commit to best practices and uphold stringent regulatory standards. This commitment not only enhances product integrity but also fosters a healthier society, encouraging consumers to make informed choices regarding their dietary supplements. By emphasizing quality and compliance, the industry can thrive, benefiting both consumers and manufacturers alike.
Frequently Asked Questions
What is the supplement manufacturing process?
The supplement manufacturing process is a systematic approach to producing dietary supplements, including vitamins, minerals, herbs, amino acids, and other health-enhancing substances.
What are the essential stages of the supplement manufacturing process?
The essential stages include sourcing raw materials, creating items, production, packaging, and distribution.
Why is maintaining high-quality standards important in the supplement manufacturing process?
Maintaining high-quality standards is crucial because it directly influences product integrity and fosters consumer confidence, ensuring that final products meet safety, efficacy, and quality standards.
What are some key features of the supplement manufacturing process?
Key features include Data Integrity Deviations, Investigations, and Standard Operating Procedures (SOPs), which ensure adherence to GXP and FDA regulations.
How does understanding the manufacturing method benefit stakeholders in the life sciences?
By understanding and refining the manufacturing method, stakeholders can effectively navigate the complexities of product development and enhance their contributions to the health and wellness industry.
