Understanding the Role of a Biotech Consultant in Life Sciences

Overview
The role of a biotech consultant in life sciences is pivotal in providing specialized advisory services that assist organizations in navigating the complexities of regulated environments. By ensuring compliance with standards such as:
- Good Manufacturing Practices (GMP)
- Quality System Regulations (QSR)
These consultants position themselves as essential partners. They conduct thorough audits, develop effective compliance adherence strategies, and enhance product development processes and quality assurance. This proactive approach not only fosters organizational integrity but also drives innovation within the industry. Ultimately, engaging with biotech consultants is a strategic move for organizations aiming to excel in compliance and operational excellence.
Introduction
The biotechnology sector is rapidly evolving, marked by stringent regulations and an escalating demand for compliance. Biotech consultants are pivotal in navigating this intricate landscape, ensuring that organizations adhere to industry standards and ensure product integrity. As businesses face mounting pressure to innovate while maintaining compliance, a critical question emerges: how can biotech consultants effectively bridge the gap between regulatory requirements and operational goals? This challenge is essential for fostering growth and trust within the life sciences industry.
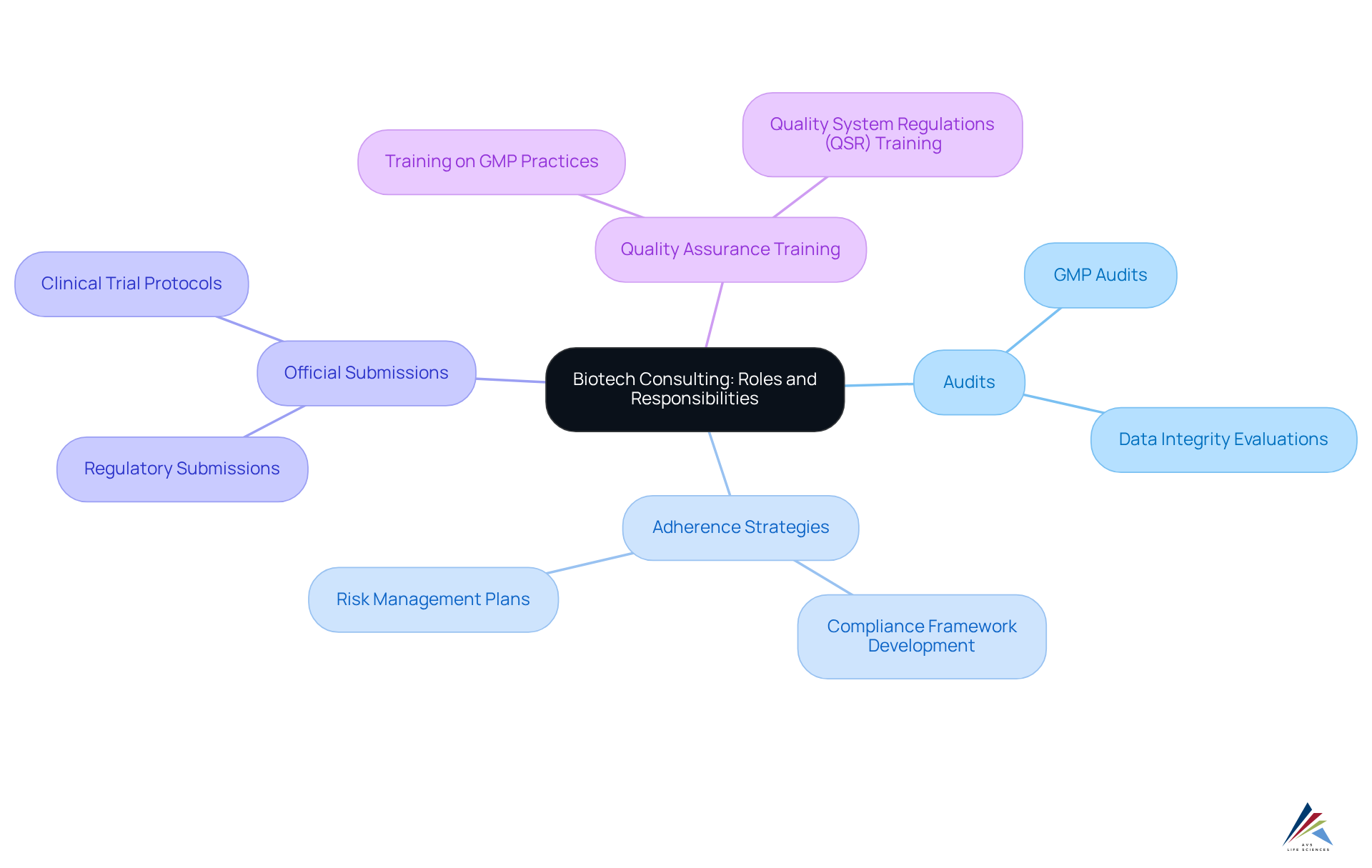
Define Biotech Consulting: Roles and Responsibilities
- Performing comprehensive audits and inspections
- Developing adherence strategies to regulatios
- Assisting with official product submissions
- Providing training on quality assurance practices
- Perform required design and operational testing
For instance, AVS Life Sciences offers extensive services, including GMP audits, Data Integrity evaluations, and support for Commissioning, Qualification and Validation (CQV). These services enhance regulatory adherence and facilitate market entry by simplifying the filing procedure and securing necessary approvals, thereby accelerating product introductions.
Statistics indicate that organizations utilizing a biotech consultant achieve superior adherence results, with many reporting substantial advancements in their oversight processes. By 2025, the importance of biotech consultants in regulatory adherence will be underscored by their ability to align business goals with market needs, ultimately fostering sustainable growth. As Paul Koziarz wisely states, "Without a regulatory framework, some organizations may not adopt any security measures at all until it is too late." This highlights the necessity of safeguarding organizational integrity and fostering innovation.
Leveraging their extensive knowledge of scientific progress and oversight frameworks, a biotech consultant—particularly one at AVS Life Sciences—enables organizations to optimize product development from initial research to market approval. This ensures that adherence is not merely a checkbox but a strategic advantage in a competitive landscape.
Contextualize Biotech Consulting: Importance in Life Sciences
The significance of a biotech consultant in the life sciences sector is becoming increasingly clear as oversight scrutiny intensifies, and compliance requirements evolve. A biotech consultant plays a pivotal role in steering organizations through complex compliance landscapes, ensuring adherence to GMPs, ISO standards, and QSRs. Their expertise is particularly vital for startups and smaller firms that often lack the in-house capabilities to effectively navigate these challenges.
Looking ahead to 2025, the demand for biotech consultant services is expected to surge, driven by the necessity for specialized expertise in regulatory leadership and development. Case studies illustrate how biotechnology advisors have successfully improved quality and safety of the drug product. A prime example is AVS Life Sciences' recent collaboration with a leading biotechnology company in San Francisco, where they facilitated the upgrade of the client's manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project encountered challenges, such as the discovery of barcode scanner cameras installed upside down, leading to anomalies in test results. AVS's meticulous documentation efforts ensured full traceability, validated by the client's quality assurance team. This partnership allowed the client to concentrate on developing targeted antibodies for cancer, ultimately improving patient outcomes.
Furthermore, as biologics continue to dominate new molecule approvals—accounting for 25% in the U.S. in 2020—the demand for services from a biotech consultant focused on these complex products is on the rise. The global biologics consulting services market is projected to expand at over 15% by 2026, highlighting the increasing demand for biotech consultants and expert guidance in this domain.
By ensuring compliance and implementing best practices, a biotech consultant not only enhances product integrity but also fosters public trust in biopharmaceutical products. Their contributions are indispensable in maintaining overall quality and safety standards within the life sciences sector, positioning them as essential partners for organizations striving to meet compliance expectations.

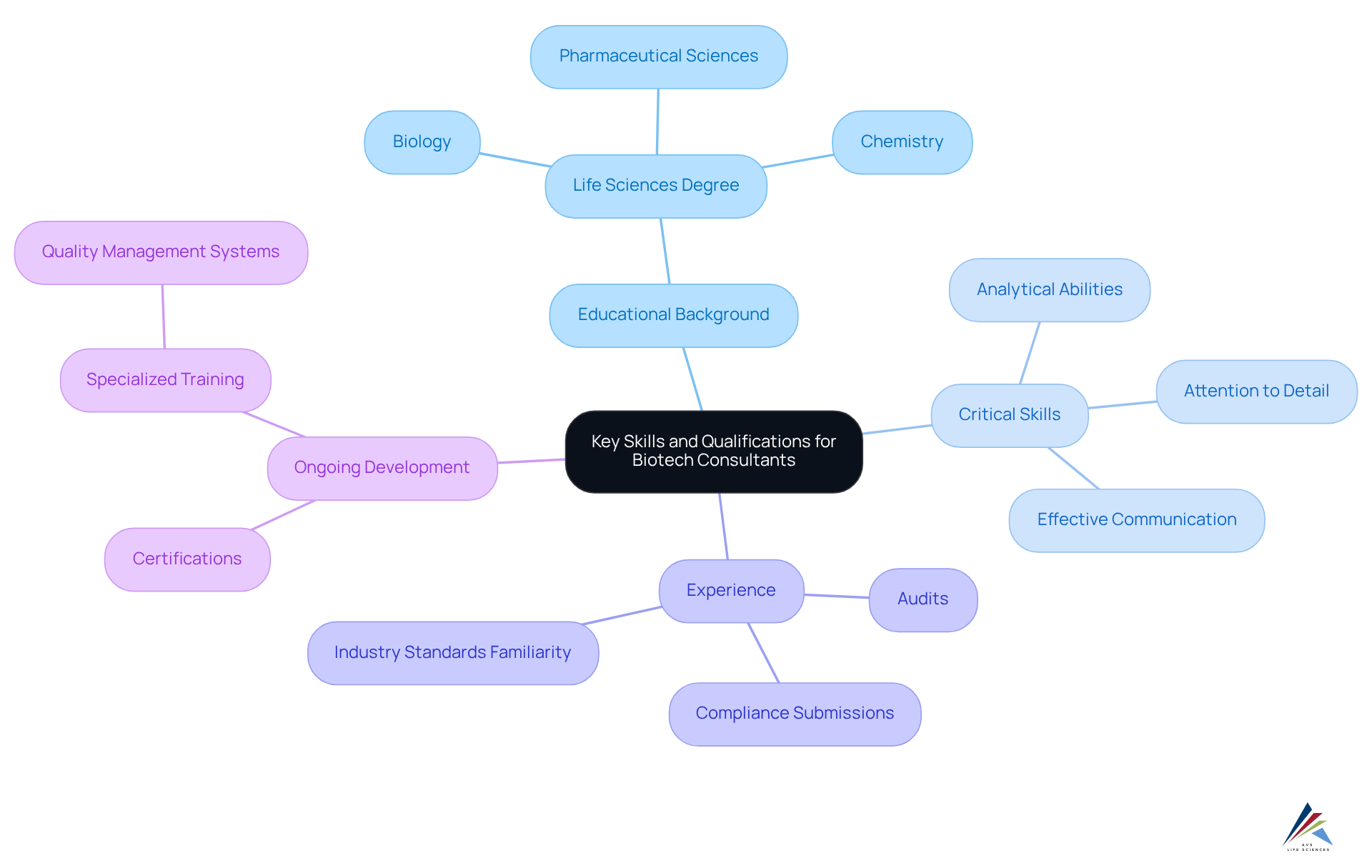
Identify Key Skills and Qualifications for Biotech Consultants
A biotech consultant must possess a robust understanding of compliance with regulations, quality assurance, quality engieering and the scientific principles underpinning biotechnology. A degree in life sciences—such as biology, chemistry, or pharmaceutical sciences—is typically essential. Beyond academic qualifications, critical skills encompass:
- Strong analytical abilities
- Meticulous attention to detail
- The capacity to communicate complex information effectively
Experience with compliance submissions and audits is crucial, along with familiarity with industry standards and guidelines.
To remain competitive, professionals should engage in ongoing career development through:
- Certifications
- Specialized training in quality management systems and quality engineering tools.
Effective training programs have been shown to enhance professionals' abilities in navigating regulatory complexities and improving operational efficiency. As the biotech landscape evolves, the demand for a biotech consultant who blends technical expertise with interpersonal skills—such as project management and communication—continues to rise, reflecting the industry's dynamic nature and the increasing complexity of product development.
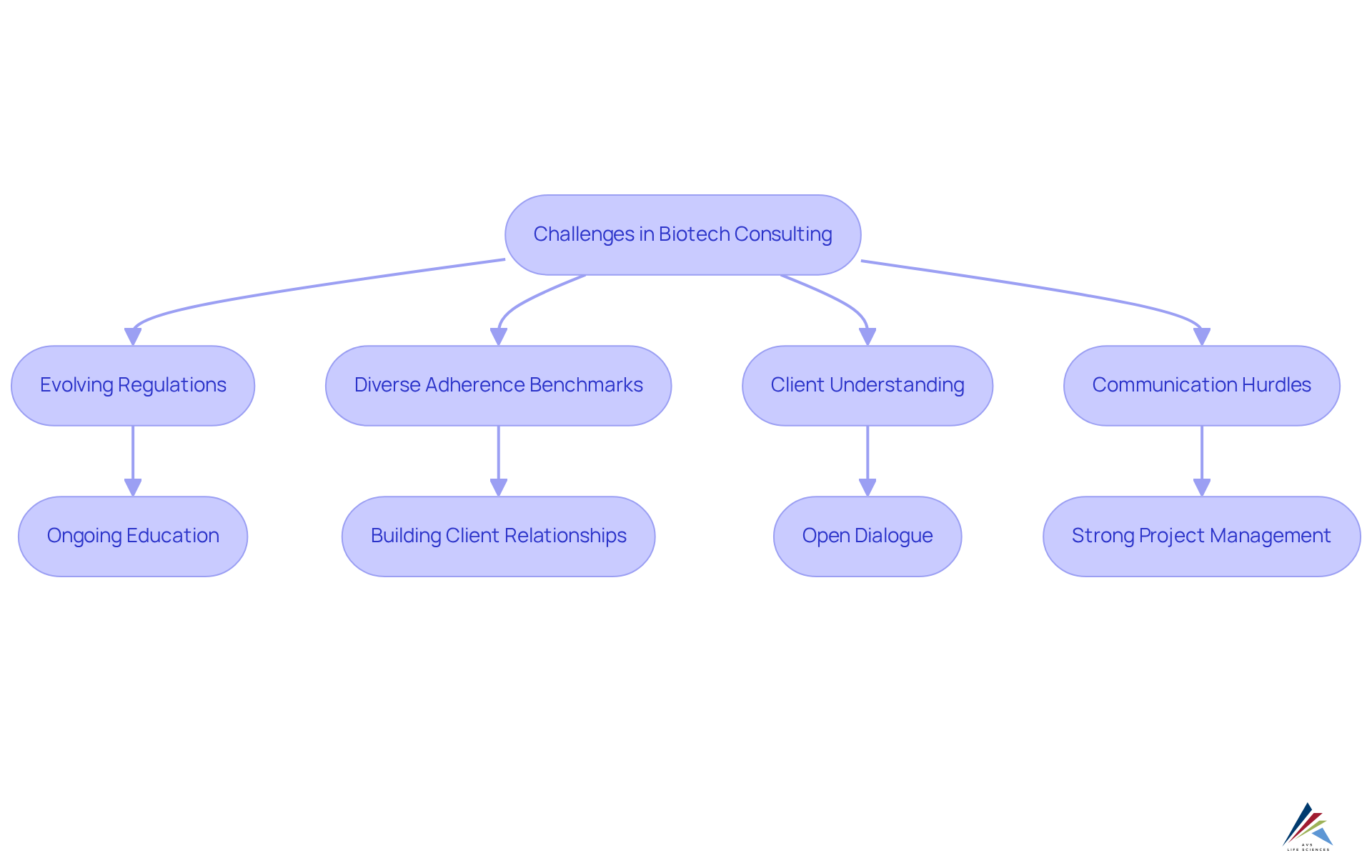
Examine Challenges and Solutions in Biotech Consulting
A biotech consultant must possess a robust understanding of compliance with regulations, quality assurance, quality engieering and the scientific principles underpinning biotechnology. A degree in life sciences—such as biology, chemistry, or pharmaceutical sciences—is typically essential. Beyond academic qualifications, critical skills encompass:
- Strong analytical abilities
- Meticulous attention to detail
- The capacity to communicate complex information effectively
Experience with compliance submissions and audits is crucial, along with familiarity with industry standards and guidelines.
To remain competitive, professionals should engage in ongoing career development through:
- Certifications
- Specialized training in quality management systems and quality engineering tools.
Effective training programs have been shown to enhance professionals' abilities in navigating regulatory complexities and improving operational efficiency. As the biotech landscape evolves, the demand for a biotech consultant who blends technical expertise with interpersonal skills—such as project management and communication—continues to rise, reflecting the industry's dynamic nature and the increasing complexity of product development.
Conclusion
The role of a biotech consultant is vital in ensuring that organizations within the life sciences sector navigate the intricate regulatory landscape effectively. By providing specialized knowledge and strategic guidance, these consultants help companies adhere to essential compliance standards, thereby transforming adherence into a competitive advantage rather than a mere obligation.
Key responsibilities of biotech consultants include:
- Conducting audits
- Developing compliance strategies
- Facilitating training on quality assurance practices
Real-world examples, such as the successful collaboration between AVS Life Sciences and a biotechnology firm, illustrate the tangible benefits of utilizing these experts. As the demand for biotech consulting services continues to rise, driven by the complexities of modern regulations and the need for innovative solutions, their contributions are becoming increasingly indispensable.
It is clear that the role of biotech consultants extends beyond mere compliance; they are essential partners in fostering innovation and ensuring public trust in biopharmaceutical products. As the life sciences landscape evolves, organizations are encouraged to recognize the value of engaging skilled consultants to not only meet regulatory requirements but also to drive sustainable growth and enhance product integrity. The future of biotechnology depends on the proactive strategies and expert guidance that these consultants provide, making their role crucial in shaping the industry’s trajectory.
Frequently Asked Questions
What is biotech consulting?
Biotech consulting involves specialized advisory services for organizations in the biotechnology and life sciences sectors, focusing on navigating compliance landscapes and ensuring adherence to regulations like Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR).
What are the primary roles of biotech consultants?
The primary roles of biotech consultants include performing comprehensive audits, developing adherence strategies, assisting with official submissions, and providing training on quality assurance practices.
What services does AVS Life Sciences offer?
AVS Life Sciences offers services such as GMP audits, Data Integrity evaluations, and support for Animal Test Facilities, which enhance regulatory adherence and facilitate market entry.
How do biotech consultants impact regulatory adherence?
Organizations utilizing biotech consultants report superior adherence results and substantial advancements in their oversight processes, which are crucial for aligning business goals with market needs.
Why is proactive regulatory compliance important in biotech?
Proactive regulatory compliance is essential as it safeguards organizational integrity and fosters innovation, preventing organizations from delaying security measures until it is too late.
How can biotech consultants optimize product development?
Biotech consultants leverage their knowledge of scientific progress and oversight frameworks to optimize product development from initial research to market approval, turning adherence into a strategic advantage.
