Understanding the Hofmeister Series for Pharmaceutical Compliance

Overview
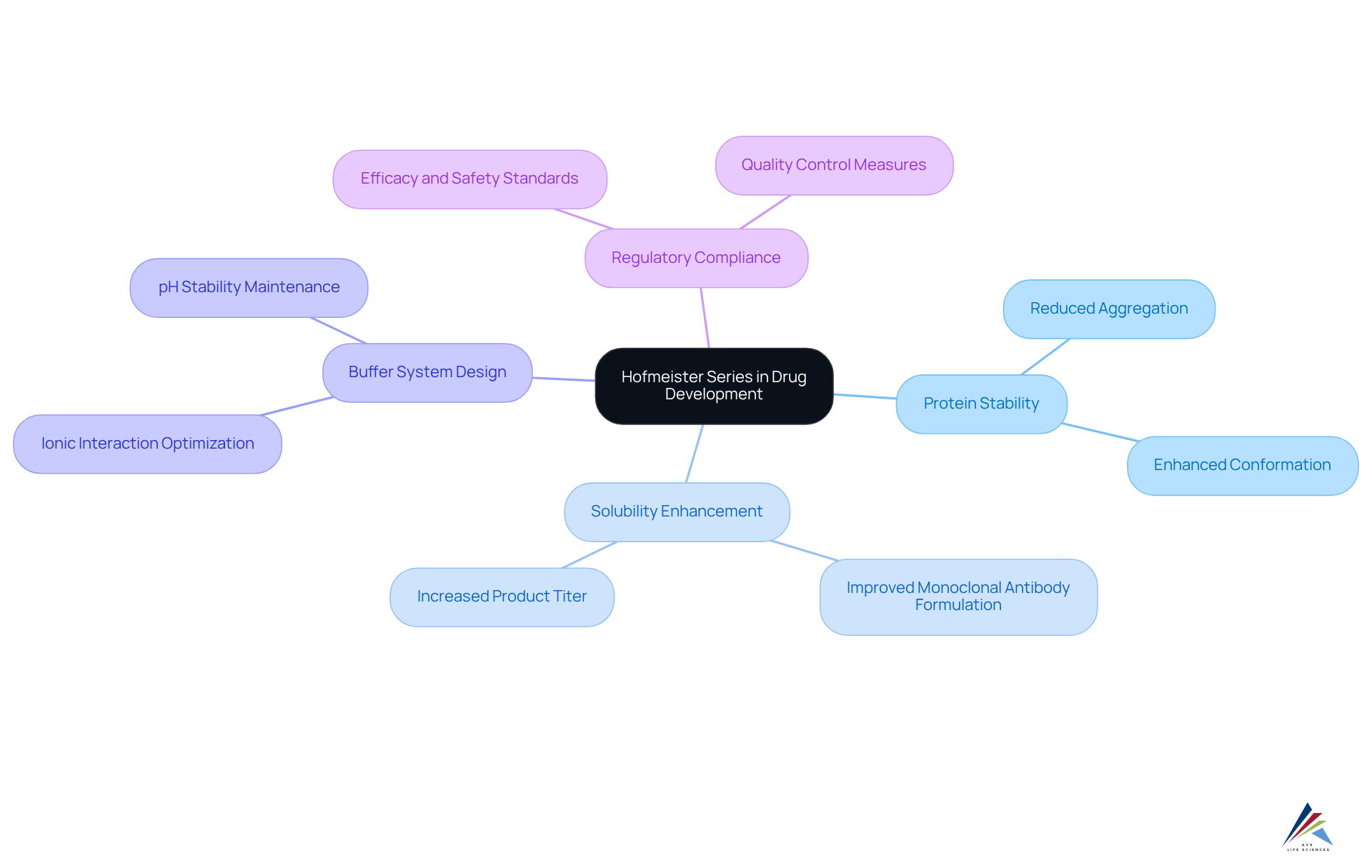
The Hofmeister series is pivotal in the realm of pharmaceutical compliance, as it categorizes charged particles according to their influence on the solubility and stability of biological macromolecules. This categorization directly affects drug formulation and efficacy. Understanding this series empowers pharmaceutical professionals to select suitable salts that bolster drug stability and adhere to regulatory standards. Such knowledge is crucial in preventing complications like aggregation, thereby ensuring product integrity.
Introduction
The Hofmeister series, a foundational concept in biochemistry established over a century ago, categorizes ions based on their effects on the solubility and stability of biological macromolecules. This classification not only enhances the understanding of drug formulation but also plays a pivotal role in ensuring pharmaceutical compliance. As the industry grapples with the challenge of maintaining product integrity and regulatory standards, a deeper grasp of the Hofmeister effects can transform drug development and quality management practices. By understanding these effects, compliance officers can implement more effective strategies that align with regulatory requirements, ultimately leading to improved product outcomes.
Define the Hofmeister Series and Its Importance in Pharmaceuticals
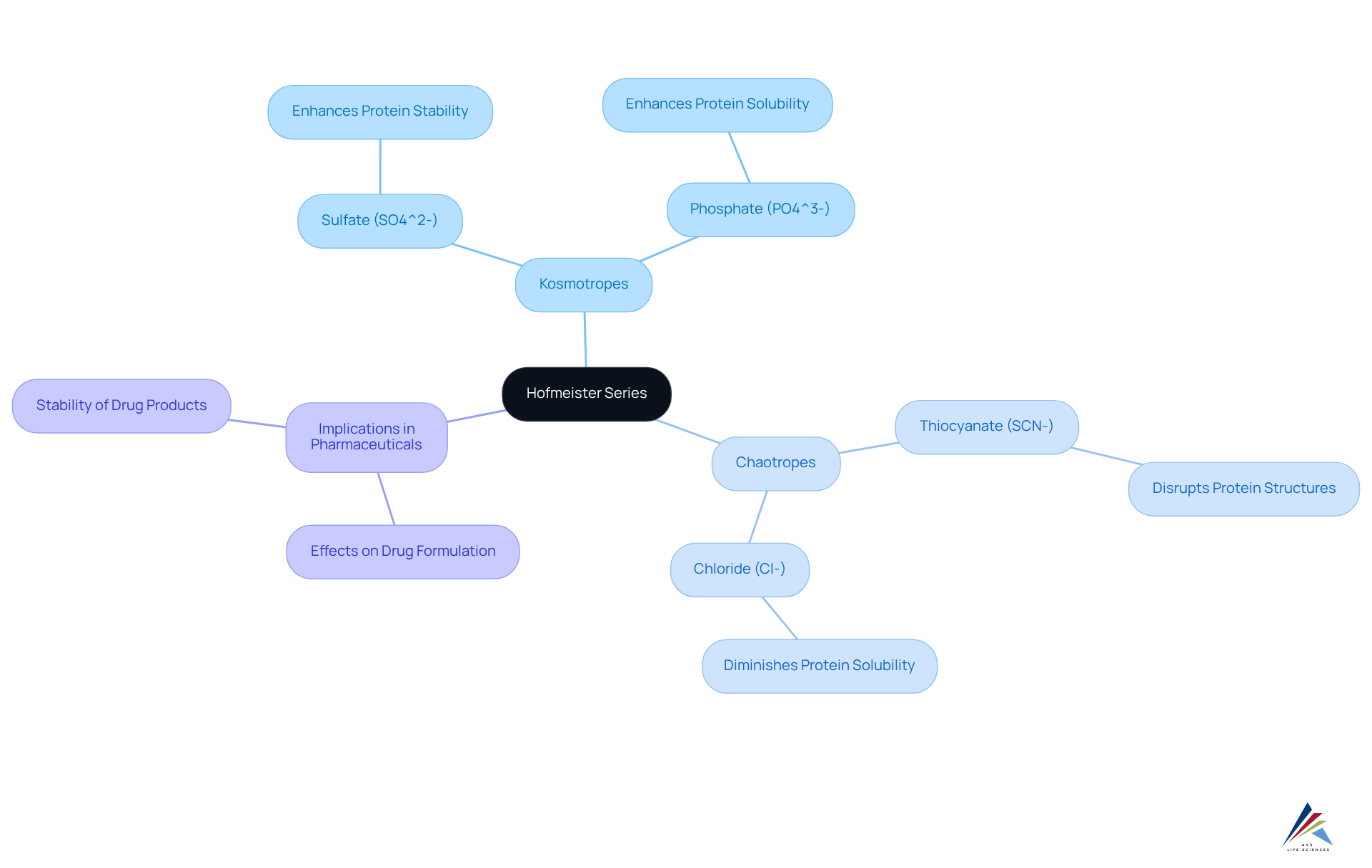
The hofmeister series, established by Franz Hofmeister in 1888, categorizes charged particles according to their impact on the solubility and stability of biological macromolecules in aqueous solutions. This series ranks particles from kosmotropes, such as sulfate (SO4^2-) and phosphate (PO4^3-), which enhance protein stability and solubility, to chaotropes, like thiocyanate (SCN-) and chloride (Cl-), which disrupt protein structures and diminish solubility.
Understanding this classification is crucial in the pharmaceutical industry, as it aids in predicting how various salts affect drug formulation, stability, and efficacy according to the . Recent studies indicate that the presence of specific charged particles can significantly affect the stability of drug formulations, with statistical analyses revealing that certain kosmotropic particles can enhance robustness by up to 30% compared to chaotropic counterparts.
For example, the incorporation of kosmotropic salts has been shown to extend the shelf life of monoclonal antibodies, whereas chaotropic salts may lead to increased aggregation and diminished efficacy. Case studies illustrate that improper particle selection, such as the use of chaotropic substances in formulations intended for consistency, can result in product instability, jeopardizing compliance with Good Manufacturing Practices (GMP) and regulatory standards.
Therefore, a thorough understanding of these interactions is imperative for ensuring adherence to regulations, including the FDA's guidelines on substance stability, and for maintaining the integrity of pharmaceutical products during evaluations.
Explore Hofmeister Effects on Biological Macromolecules
The Hofmeister series illustrates the significant influence of various charged particles on the durability and solubility of large molecules. Kosmotropic substances, such as sulfate and phosphate, enhance the stability of these molecules by promoting hydration and facilitating proper folding. Conversely, chaotropic particles like iodide and thiocyanate disrupt hydrogen bonding and hydration shells, leading to denaturation and aggregation of polypeptides.
These interactions hold critical importance in pharmaceutical contexts, as they directly impact , bioavailability, and therapeutic efficacy. For example, certain salts can either boost or inhibit enzyme activity, which is essential for effective drug formulations. By understanding these ion-specific interactions as described by the Hofmeister series, pharmaceutical professionals can develop more effective and stable drug products, ultimately improving patient outcomes.
Historically, the foundation for comprehending these essential interactions was established in 1888 when a scientist created a collection of charged particles while examining the salt concentration needed to precipitate proteins from egg white.
Integrate Hofmeister Insights into Regulatory Compliance and Quality Management
Incorporating insights from the Series into regulatory compliance and quality management is essential for pharmaceutical companies. A comprehensive evaluation of the charged particles used in formulations is critical, as their arrangement within the hofmeister series can significantly influence the selection of salts that enhance both robustness and dissolvability. This meticulous selection minimizes the risks of aggregation or denaturation, which are vital for maintaining product integrity.
To ensure adherence to (GMP) and other regulatory standards, companies must establish robust testing protocols that evaluate the impact of various ions on product consistency throughout the development lifecycle. Regular audits and documentation reviews should encompass assessments of ion-specific effects, enabling organizations to proactively address potential compliance issues. By concentrating on these strategies, pharmaceutical companies can fortify their quality management systems and diminish the risk of non-compliance during regulatory inspections.
Insights from industry studies reveal that companies prioritizing material issues can achieve up to 50% increased profit from sustainability efforts, underscoring the necessity of aligning quality management with broader corporate strategies. Furthermore, case studies such as the Rio Tinto incident highlight the repercussions of neglecting regulatory compliance, reinforcing the need for thorough assessments and proactive measures in quality management. By integrating these insights, pharmaceutical companies can enhance their compliance frameworks and ensure sustainable practices.
Practical Applications of the Hofmeister Series in Drug Development
The series plays a crucial role in drug development, particularly in the formulation of protein-based therapeutics. Understanding enables pharmaceutical companies to select excipients that enhance stability and solubility. For instance, a biopharmaceutical company effectively utilized the hofmeister series to enhance a monoclonal antibody formulation, resulting in improved solubility and reduced aggregation during storage. Furthermore, insights from the hofmeister series inform the design of buffer systems in purification processes, ensuring that selected ions support the desired protein conformation and activity. This strategic application not only enhances the efficacy and safety of pharmaceutical products but also aids in maintaining compliance with regulatory standards. Recent studies indicate that utilizing the hofmeister series can significantly reduce aggregation rates and improve overall product stability, showcasing its value in the biopharmaceutical landscape.
Conclusion
Understanding the Hofmeister series is essential for the pharmaceutical industry, as it provides a framework for predicting how different charged particles affect drug formulation, stability, and efficacy. By categorizing ions into kosmotropes and chaotropes, this classification system empowers pharmaceutical professionals to make informed decisions that enhance product integrity and ensure compliance with regulatory standards.
Key insights throughout the article highlight the profound impact of charged particles on biological macromolecules, emphasizing the critical importance of selecting the right ions to improve drug solubility and therapeutic efficacy. The presented case studies demonstrate how the strategic use of kosmotropic salts can significantly enhance the stability of formulations, while neglecting these principles can lead to product instability and regulatory challenges. Furthermore, integrating Hofmeister insights into quality management practices is vital for maintaining compliance and achieving sustainable outcomes in drug development.
Ultimately, leveraging the Hofmeister series not only aids in creating effective and stable pharmaceutical products but also reinforces the significance of regulatory compliance in the industry. By prioritizing the understanding and application of these principles, pharmaceutical companies can enhance their product development processes, ensuring they deliver safe and effective therapies while adhering to the highest standards of quality and compliance. Embracing this knowledge is not merely beneficial; it is a necessity for advancing pharmaceutical innovation and safeguarding patient health.
Frequently Asked Questions
What is the Hofmeister Series?
The Hofmeister Series, established by Franz Hofmeister in 1888, categorizes charged particles based on their effects on the solubility and stability of biological macromolecules in aqueous solutions. It ranks particles from kosmotropes, which enhance stability and solubility, to chaotropes, which disrupt structures and reduce solubility.
What are kosmotropes and chaotropes?
Kosmotropes are charged particles, such as sulfate (SO4^2-) and phosphate (PO4^3-), that enhance protein stability and solubility. Chaotropes, like thiocyanate (SCN-) and chloride (Cl-), disrupt protein structures and diminish solubility.
Why is the Hofmeister Series important in pharmaceuticals?
The Hofmeister Series is crucial in the pharmaceutical industry as it helps predict how different salts affect drug formulation, stability, and efficacy. Understanding these interactions is essential for maintaining product integrity and compliance with regulations.
How do kosmotropic and chaotropic particles affect drug formulations?
Kosmotropic particles can enhance the robustness of drug formulations by up to 30%, extending the shelf life of products like monoclonal antibodies. In contrast, chaotropic particles may lead to increased aggregation and reduced efficacy, compromising product stability.
What are the consequences of improper particle selection in drug formulations?
Using chaotropic substances in formulations intended for consistency can result in product instability, potentially jeopardizing compliance with Good Manufacturing Practices (GMP) and regulatory standards.
How does the Hofmeister Series relate to regulatory guidelines?
A thorough understanding of the Hofmeister Series interactions is imperative for adhering to regulations, including the FDA's guidelines on substance stability, ensuring the integrity of pharmaceutical products during evaluations.
