Understanding the FSP Clinical Research Model: Key Features and Benefits

Overview
The FSP clinical research model represents a strategic outsourcing framework that empowers pharmaceutical companies to retain control over clinical trials while delegating specific functions to specialized service providers. This approach significantly enhances operational efficiency and flexibility. Key benefits include:
- Reduced costs
- Improved data management
- Access to specialized expertise
These advantages have led to a notable increase in the adoption of this model within the industry, underscoring its relevance in addressing contemporary clinical research challenges.
Introduction
The landscape of clinical research is evolving rapidly, driven by the need for efficiency and specialization in drug development. At the forefront of this evolution is the Functional Service Provider (FSP) model, a strategic outsourcing solution that empowers pharmaceutical companies to maintain control while leveraging expert services for specific trial components.
As organizations navigate the complexities of medical studies, they face pressing compliance challenges. How can the FSP model not only enhance operational effectiveness but also ensure adherence to ever-changing regulatory standards?
This article delves into the key features, benefits, and strategic importance of the FSP framework, revealing its pivotal role in shaping the future of clinical research.
Define the FSP Clinical Research Model
The FSP clinical research framework represents a strategic outsourcing method where pharmaceutical firms or research entities collaborate with specialized service providers to manage specific aspects of clinical studies. This approach distinguishes itself from traditional full-service outsourcing, which typically sees a single provider overseeing all trial components. Instead, the FSP clinical research model empowers sponsors to maintain control over the entire project while delegating critical tasks—such as data management, biostatistics, and regulatory affairs—to experts. This flexibility not only allows organizations to according to specific project needs but also enhances operational efficiency and effectiveness in development.
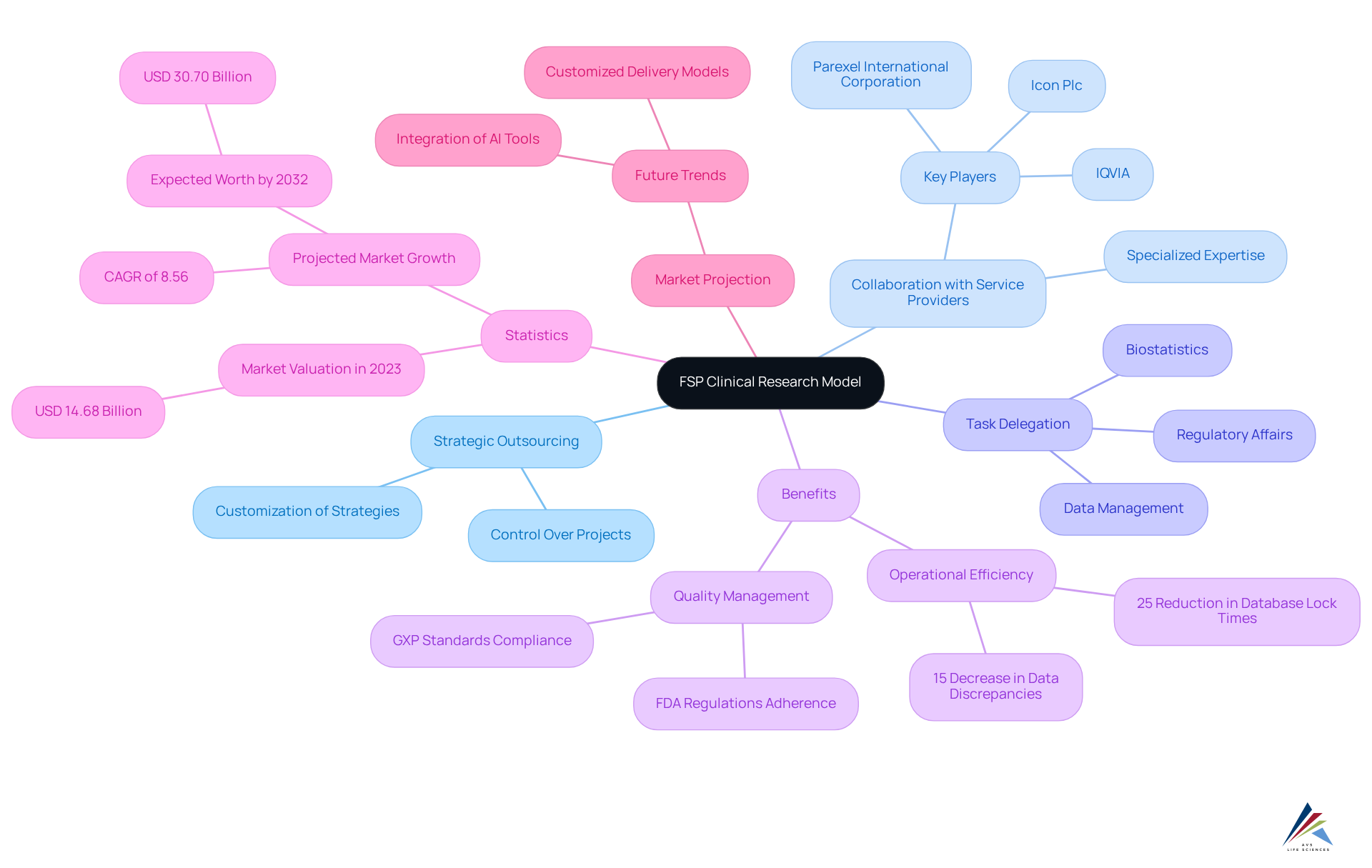
AVS Life Sciences emphasizes comprehensive quality management and regulatory compliance solutions, ensuring adherence to GXP standards and FDA regulations throughout the research process. Statistics reveal:
- A 25% reduction in database lock times
- A 15% decrease in data discrepancies for companies that have embraced this approach
Furthermore, the FSP market is projected to expand significantly, with an estimated worth of USD 30.70 billion by 2032, reflecting the increasing reliance on specialized providers to navigate the complexities of medical studies. As the landscape of medication development evolves, the FSP clinical research approach emerges as an essential strategy for organizations aiming to enhance their research processes while ensuring compliance with regulatory standards. Additionally, AVS Life Sciences implements Great Documentation Practices and Standard Operating Procedures (SOPs) to bolster quality management and facilitate effective interactions with the FDA.
Trace the Evolution of the FSP Model in Clinical Research
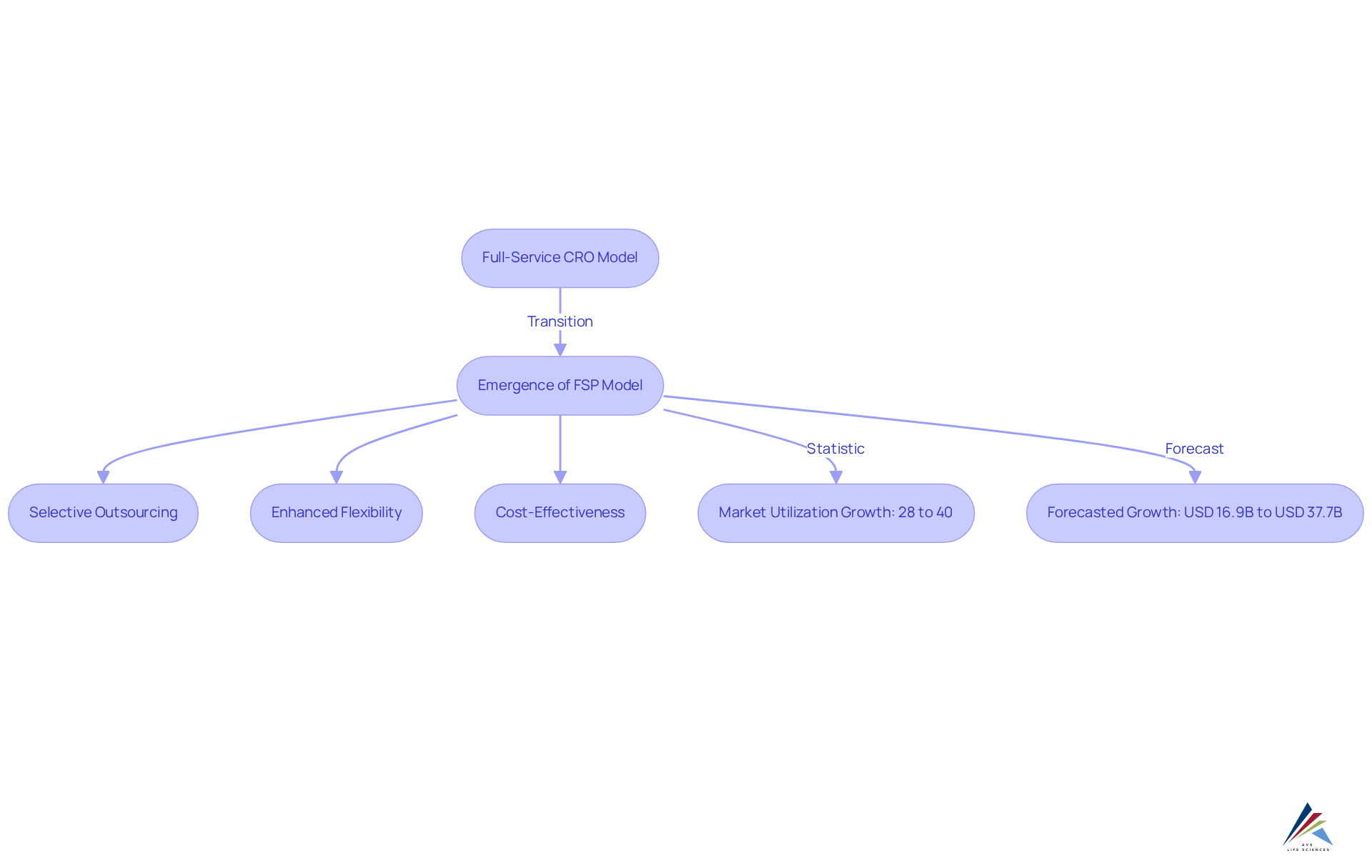
Over the last two decades, the Functional Service Provider (FSP) approach has undergone significant evolution, primarily driven by the increasing complexity of medical studies and the demand for enhanced flexibility in resource management. Initially, full-service contract research organizations (CROs) managed research trials, overseeing all facets of trial execution. However, as the pharmaceutical industry grappled with escalating costs and stringent regulatory requirements, the FSP framework emerged as a compelling alternative. This innovative model empowers sponsors to while retaining oversight and control, facilitating swift adaptation to changing project demands and optimized resource allocation.
The rise of the FSP clinical research framework reflects a broader trend towards specialization and efficiency within the field. The market utilization of FSP services surged from 28% in 2018 to over 40% in 2021, signaling a growing recognition of its benefits. Moreover, the FSP market is anticipated to expand from USD 16.9 billion in 2024 to USD 37.7 billion by 2034, with an expected compound annual growth rate (CAGR) of 8.4% during the forecast period of 2025-2034. As organizations pursue cost-effective solutions and enhanced data control, the FSP clinical research model has gained traction, attracting a diverse clientele that includes both startups and large pharmaceutical firms. Currently, approximately 4,000 research studies are underway, underscoring the active demand for FSP services within the sector. This transformation not only enhances operational flexibility but also aligns with the evolving needs of the industry, positioning FSP clinical research as pivotal players in the future of trial management.
Examine Key Features and Operational Mechanisms of the FSP Model
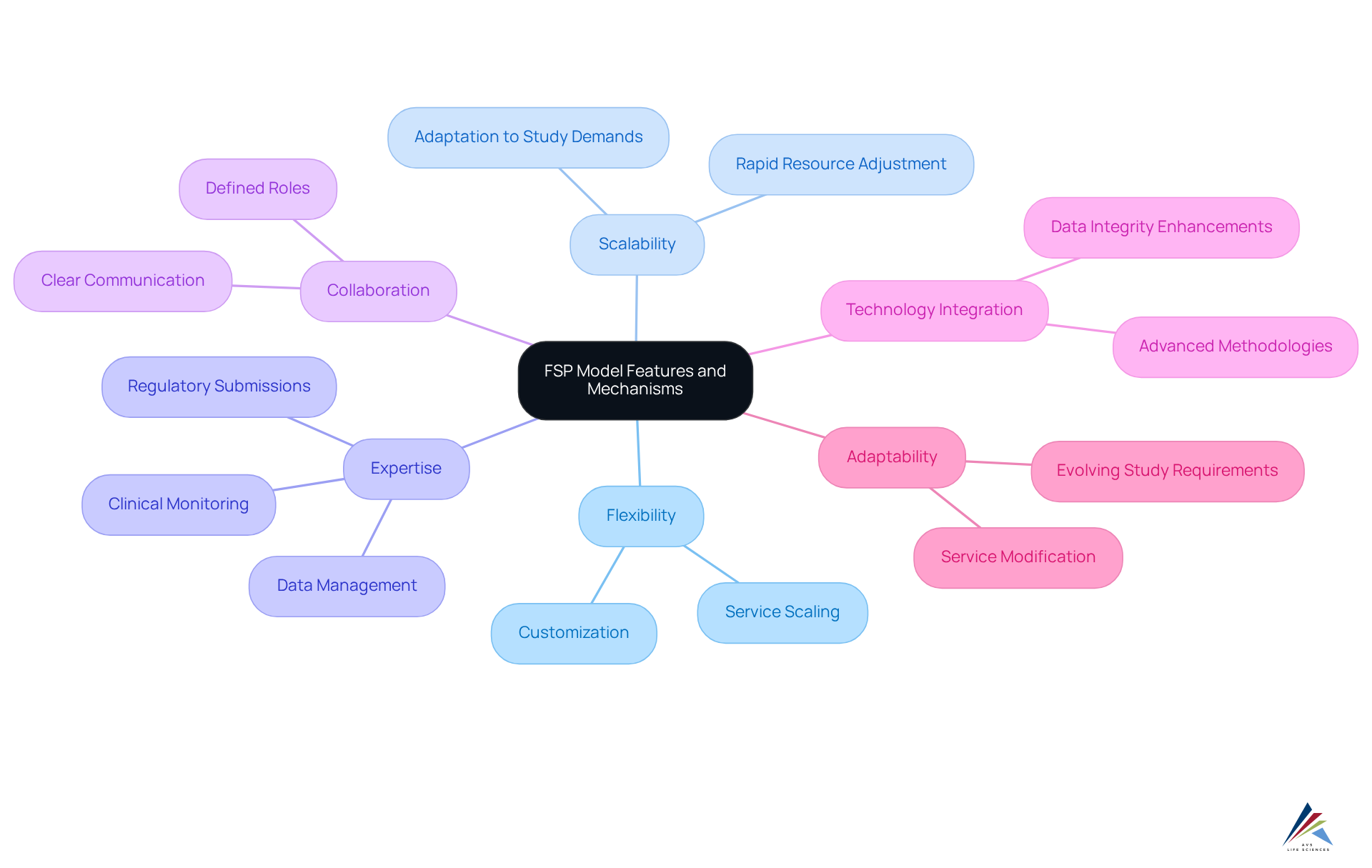
The FSP clinical research framework is distinguished by its inherent flexibility, scalability, and specialized expertise, enabling organizations to effectively outsource critical functions such as clinical monitoring, data management, and regulatory submissions. This focused approach empowers sponsors to hone in on their core competencies while capitalizing on the strengths of their FSP partners.
Operationally, the FSP framework flourishes through a collaborative partnership between the sponsor and the service provider, where clear communication and well-defined roles are essential. This synergy not only enables sponsors to retain control over vital study components but also allows them to leverage the offered by FSP clinical research.
Notably, this model often integrates advanced technologies and methodologies, which enhance efficiency and ensure data integrity throughout the research process. For instance, a U.S.-based biopharmaceutical firm reported a remarkable 25% reduction in database lock times and a 15% decrease in data discrepancies after outsourcing data management to an FSP. Such outcomes underscore the effectiveness of specialized knowledge in optimizing research operations.
Furthermore, the adaptability of the FSP clinical research framework addresses the evolving requirements of clinical studies, allowing sponsors to modify services as needed, ultimately leading to improved project results.
Explore Benefits and Strategic Importance of the FSP Model for Pharmaceutical Professionals
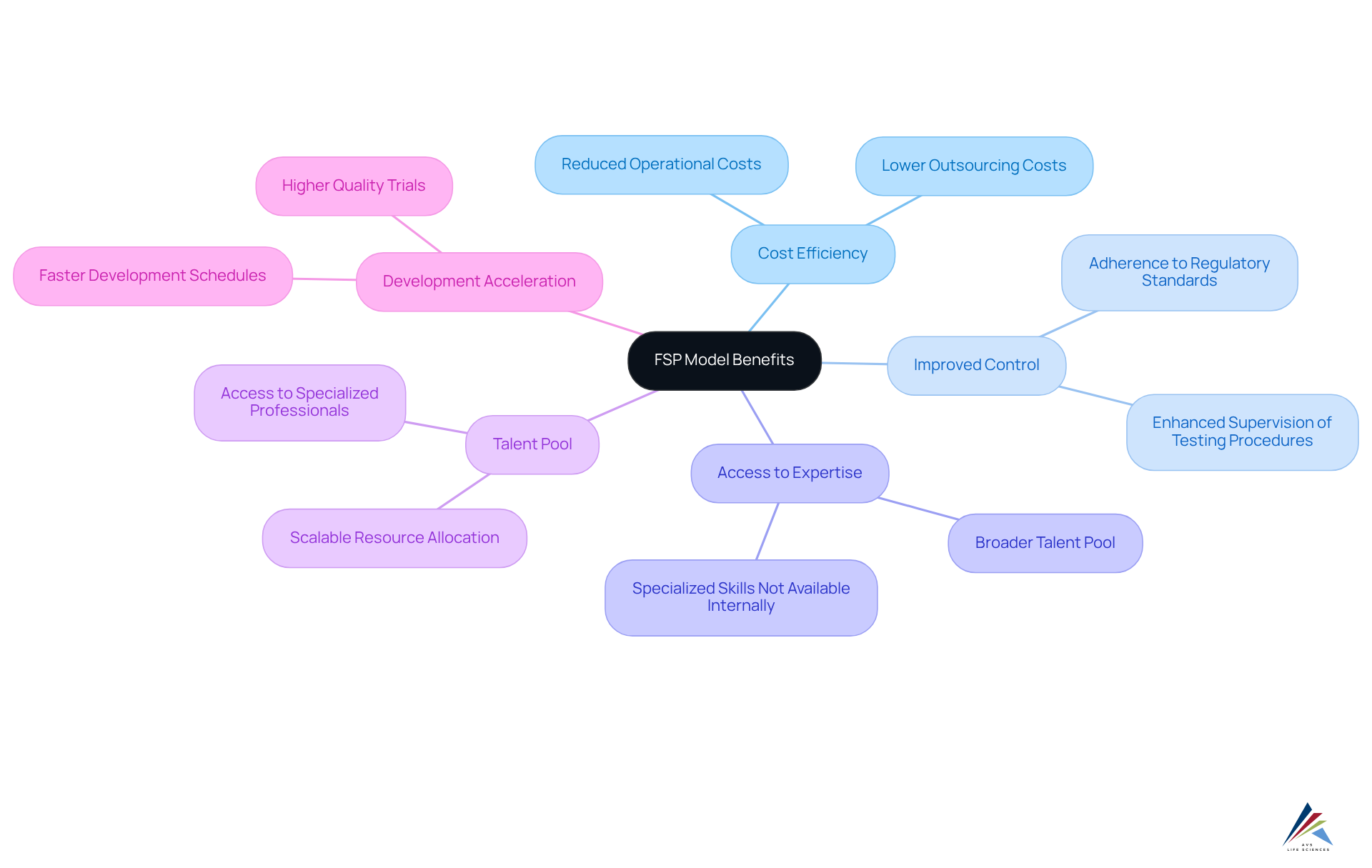
The FSP clinical research approach provides substantial benefits for pharmaceutical professionals, particularly in terms of cost efficiency, improved control, and access to specialized expertise. By outsourcing specific functions, organizations can effectively reduce operational costs and optimize resource allocation. This framework facilitates enhanced supervision of testing procedures, ensuring adherence to regulatory standards while maintaining high-quality data management.
Furthermore, the FSP framework allows companies to tap into a broader talent pool, accessing specialized skills that may not be available internally. This strategic method not only but also elevates the overall quality of research trials.
Recent trends underscore this shift, with the adoption of FSP models surging, as utilization rates increased from 28% in 2018 to over 40% in 2021. This statistic reflects a growing recognition of their value in navigating the complexities of drug development.
Case studies further exemplify the effectiveness of FSP clinical research partnerships, demonstrating how tailored solutions can enhance operational control and drive successful outcomes.
Conclusion
The FSP clinical research model signifies a transformative approach within the pharmaceutical industry, empowering organizations to strategically outsource specific functions while maintaining oversight and control. This model not only enhances operational efficiency but also enables sponsors to leverage specialized expertise, ultimately resulting in more effective and compliant clinical trials.
Key features of the FSP model have been explored, including its flexibility, scalability, and collaborative nature. The substantial benefits of adopting this framework—such as reduced operational costs, improved data management, and accelerated development timelines—underscore its growing significance in the evolving landscape of clinical research. Furthermore, the significant market growth projections highlight the increasing reliance on FSP services as organizations navigate the complexities of drug development.
In light of these insights, embracing the FSP clinical research model emerges as a crucial strategy for pharmaceutical professionals seeking to enhance their research processes. By tapping into specialized resources while maintaining a focus on core competencies, organizations can not only improve their operational outcomes but also position themselves for success in an increasingly competitive market. Engaging with this model is not merely a response to current industry demands; it represents a proactive step toward future-proofing clinical research endeavors.
Frequently Asked Questions
What is the FSP clinical research model?
The FSP clinical research model is a strategic outsourcing method where pharmaceutical firms or research entities collaborate with specialized service providers to manage specific aspects of clinical studies, allowing sponsors to maintain control while delegating critical tasks.
How does the FSP model differ from traditional outsourcing?
Unlike traditional full-service outsourcing, where a single provider oversees all trial components, the FSP model allows organizations to customize their outsourcing strategies by delegating specific tasks to experts while retaining overall project control.
What are some key benefits of the FSP clinical research model?
Key benefits include enhanced operational efficiency, the ability to customize outsourcing strategies according to project needs, and improved adherence to quality management and regulatory compliance.
What statistics support the effectiveness of the FSP model?
Statistics indicate a 25% reduction in database lock times and a 15% decrease in data discrepancies for companies that have adopted the FSP approach.
What is the projected market worth of the FSP model by 2032?
The FSP market is projected to be worth approximately USD 30.70 billion by 2032, reflecting the growing reliance on specialized providers in clinical research.
How does AVS Life Sciences ensure quality management in clinical research?
AVS Life Sciences emphasizes comprehensive quality management and regulatory compliance solutions, ensuring adherence to GXP standards and FDA regulations, and implements Great Documentation Practices and Standard Operating Procedures (SOPs).
