Understanding the CMC Acronym: Importance in Pharmaceuticals

Overview
The CMC acronym, representing Chemistry, Manufacturing, and Controls, plays a pivotal role in the pharmaceutical industry by ensuring the quality, safety, and efficacy of drug products throughout their development and lifecycle. Inadequate CMC documentation stands as a primary cause of failed drug submissions, highlighting the pressing need for rigorous compliance with regulatory standards. This compliance is essential not only for securing marketing authorization for new therapies but also for maintaining the integrity of the pharmaceutical supply chain. Engaging with AVS Life Sciences can provide the necessary solutions to navigate these challenges effectively.
Introduction
Understanding the complexities of drug development is crucial, particularly when considering the Chemistry, Manufacturing, and Controls (CMC) acronym. This multifaceted term signifies not only the backbone of pharmaceutical quality assurance but also underscores the rigorous documentation and processes necessary to ensure safety and efficacy.
With over 40% of failed Investigational New Drug submissions attributed to CMC-related issues, the stakes are undeniably high. Pharmaceutical companies face significant challenges in navigating these critical regulatory requirements. To enhance their chances of success, they must effectively leverage CMC practices.
What strategies can be employed to overcome these hurdles and achieve compliance? The path forward is not just about understanding CMC; it is about mastering it.
Define CMC: Meaning and Significance in Pharmaceuticals
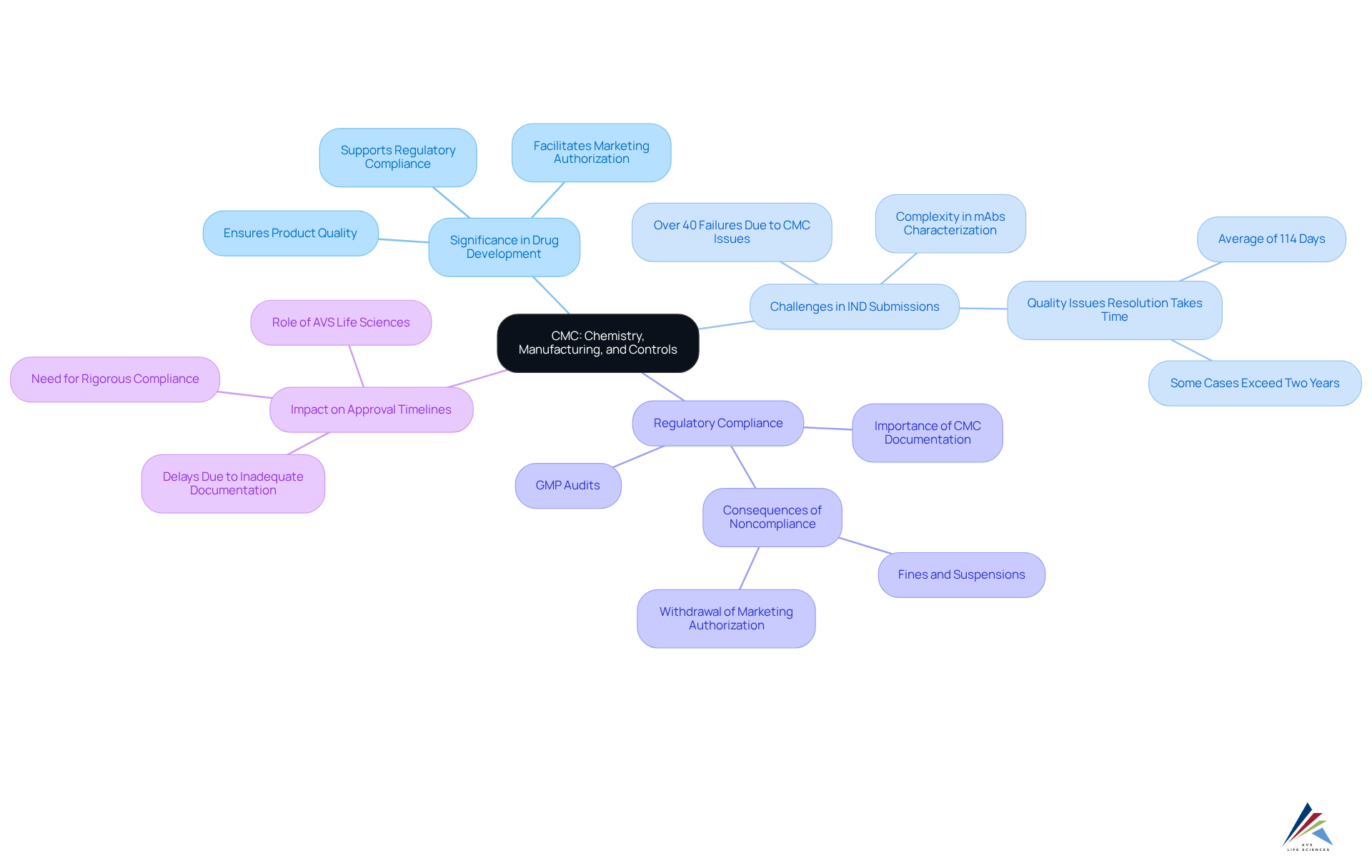
The cmc acronym refers to Chemistry, Manufacturing, and Controls, which encompasses a comprehensive array of activities and documentation that are vital for ensuring the excellence and uniformity of pharmaceutical products throughout their lifecycle. This includes the development of medication formulations, manufacturing processes, and the stringent controls necessary to guarantee that products meet rigorous quality standards.
The CMC acronym underscores its significance in drug development, where it ensures that final products are safe, effective, and compliant with regulatory requirements. Notably, over 40% of unsuccessful Investigational New Drug (IND) submissions for oncology therapies stem from challenges associated with the cmc acronym, highlighting the critical need for thorough CMC documentation and management.
Furthermore, the CMC acronym is essential for regulatory submissions and for maintaining standards post-approval, establishing it as a cornerstone of pharmaceutical development. Inadequate or incomplete CMC documentation can result in substantial delays in approval timelines or even outright rejections, further emphasizing the necessity of rigorous compliance.
As pharmaceutical development grows increasingly complex, the role of the cmc acronym in ensuring consistency and reliability in the manufacturing process becomes ever more crucial, ultimately aiding sponsors in securing marketing authorization for transformative therapies.
AVS Life Sciences, a leading provider of management and regulatory compliance solutions, emphasizes the importance of incorporating the cmc acronym into pharmaceutical development to ensure excellence. Their services, including , are vital for navigating the complexities associated with the CMC acronym considerations and ensuring adherence to regulatory standards.
Trace the Evolution of CMC in Pharmaceutical Development
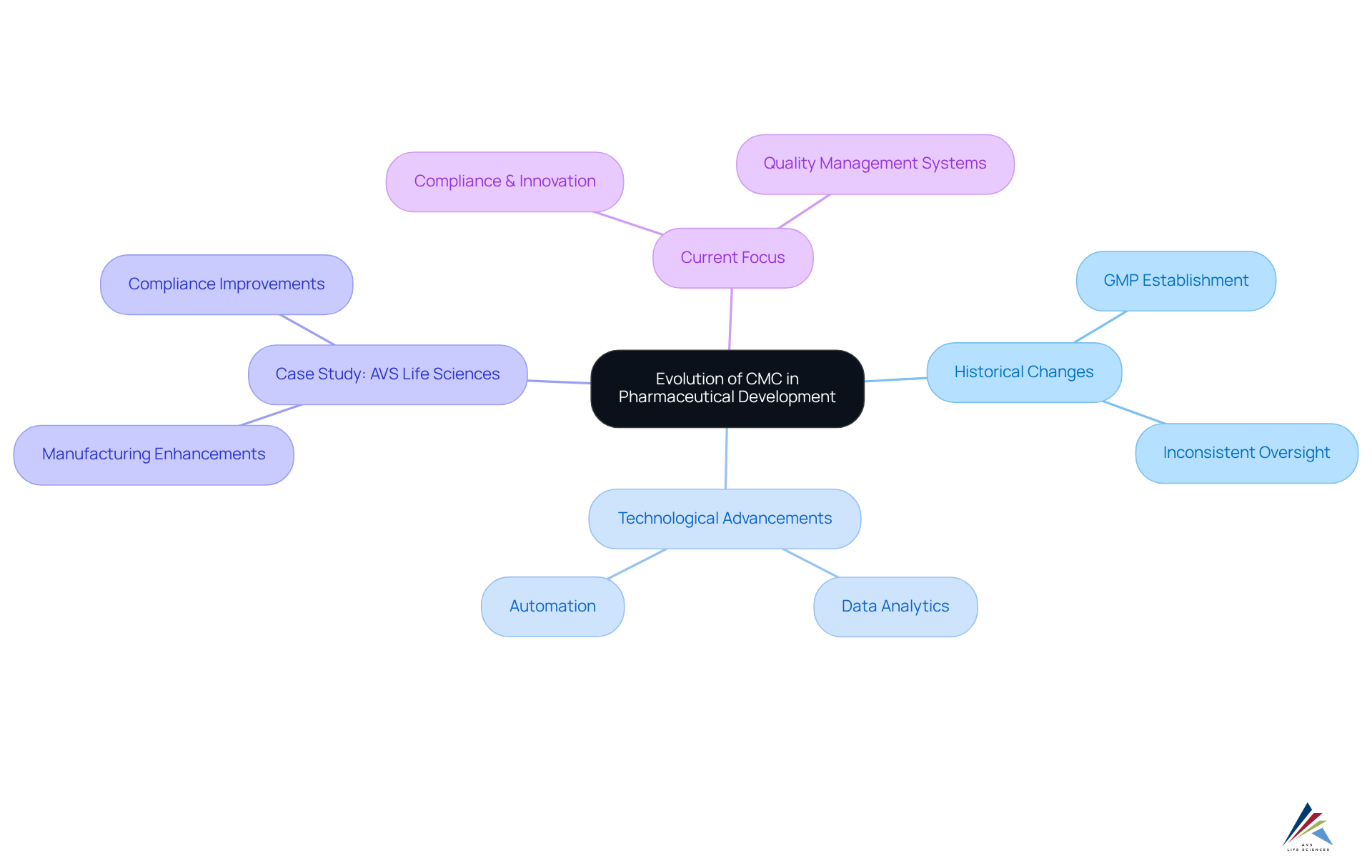
The evolution of the cmc acronym in Chemistry, Manufacturing, and Controls (CMC) within pharmaceutical development signifies a critical transformation from the early stages of drug production, characterized by inconsistent oversight. As the pharmaceutical sector expanded and regulatory frameworks emerged, the demand for standardized practices became increasingly evident. The establishment of Good Manufacturing Practices (GMP) in the mid-20th century marked a significant turning point, instituting rigorous standards that manufacturers must follow to guarantee product safety and efficacy.
A compelling illustration of this transformation is found in AVS Life Sciences' recent partnership with a prominent biotechnology firm in San Francisco. AVS adeptly upgraded their manufacturing facility from a Biosafety Level 1 GMP setting to a Level 2 GMP environment, achieving project completion on schedule and within budget. This enhancement not only bolstered the facility's compliance with regulatory standards but also refined assurance processes, allowing the client to focus on developing targeted antibodies for cancer treatment. The methodologies employed during this upgrade involved comprehensive gap analysis, meticulous documentation for traceability, and the final installation of equipment, which were crucial for the client’s assurance team.
Over the years, technological advancements and a deeper understanding of drug formulation have propelled the evolution of CMC practices. The integration of data analytics and automation has significantly improved assurance while streamlining manufacturing processes, leading to enhanced efficiency and reliability. In the case of AVS Life Sciences, their rigorous documentation efforts demonstrated complete traceability, essential for the client’s assurance team. This experience underscores the importance of continuous assessment and enhancement in control processes, particularly when inconsistencies in test results necessitated a reevaluation of business procedures and responsibilities within the client's QC laboratory team. Valuable lessons derived from these anomalies sparked discussions regarding team workload and responsibilities, highlighting the necessity for dependable testing protocols.
Currently, the CMC acronym represents a dual focus on compliance and innovation, empowering companies to adeptly navigate the complexities of regulatory environments while consistently elevating the quality of their offerings. This evolution emphasizes the pivotal role of the cmc acronym in ensuring that pharmaceutical products meet the .
Identify Key Characteristics of Effective CMC Practices
Effective CMC practices, often referred to by the CMC acronym, are characterized by several key elements:
- Thorough documentation
- Robust quality control measures
- Proactive approach to risk management
Documentation is vital; it offers a traceable account of all processes and decisions made during product development, including the essential phases of computer system validation (CSV) such as Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Quality control measures—including regular audits and testing—ensure that products consistently meet established standards in line with GXP and FDA regulations. Furthermore, a proactive risk management strategy enables organizations to identify potential compliance issues before they arise, facilitating timely corrective actions. Companies that excel in CMC often foster a culture of continuous improvement, regularly updating their practices to align with evolving regulatory expectations and technological advancements. This commitment not only ensures compliance with (GMP) but also maintains data integrity.

Examine the Regulatory Importance of CMC in Drug Development
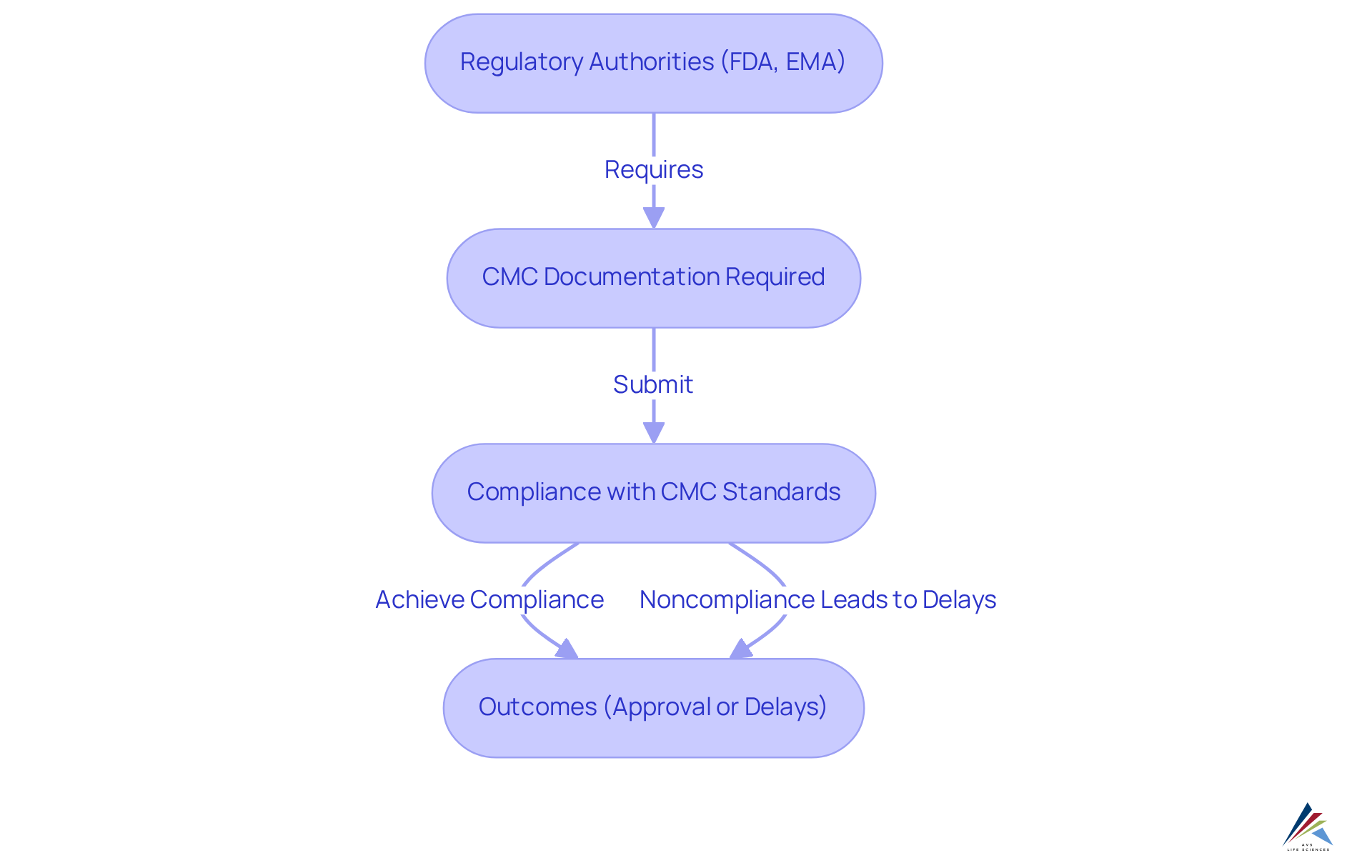
The regulatory importance of the CMC acronym, representing Chemistry, Manufacturing, and Controls, in pharmaceutical development cannot be overstated. Regulatory authorities, such as the FDA and EMA, mandate comprehensive documentation known as the CMC acronym as a prerequisite for the approval of pharmaceuticals. This documentation encompasses the product's formulation, manufacturing processes, and . Noncompliance with the CMC acronym requirements can lead to significant delays in approval timelines.
Studies indicate that over 40% of unsuccessful Investigational New Drug (IND) filings for oncology drugs arise from CMC-related issues, often due to insufficient regulatory experience among sponsors. Such setbacks not only escalate expenses but may also result in recalls and jeopardize market approval. Continuous adherence to the CMC acronym standards is essential for sustaining market presence.
Therefore, organizations must integrate the CMC acronym considerations into their development strategies, ensuring they meet regulatory expectations while delivering safe and effective products to the market.
Conclusion
The acronym CMC, which stands for Chemistry, Manufacturing, and Controls, is a cornerstone of the pharmaceutical industry, ensuring that drug products are developed with the highest standards of quality and compliance. Its importance is paramount, as it encompasses essential activities and documentation necessary for maintaining safety, efficacy, and adherence to regulatory requirements throughout the product lifecycle.
This article highlights the evolution of CMC practices, the critical nature of thorough documentation, and the necessity of robust quality control measures. Notably, it underscores that inadequate CMC management can lead to significant setbacks, particularly in regulatory submissions, where over 40% of unsuccessful Investigational New Drug filings are linked to CMC-related issues. Moreover, the integration of modern technologies has revolutionized CMC practices, fostering enhanced compliance and innovation within the pharmaceutical sector.
Ultimately, CMC is not merely a regulatory obligation; it serves as a foundational element that upholds the integrity of pharmaceutical development. By prioritizing effective CMC strategies, organizations can adeptly navigate the complexities of regulatory environments while ensuring the delivery of safe and effective therapies to patients. Embracing these practices is crucial for cultivating a culture of continuous improvement and achieving long-term success in the competitive pharmaceutical landscape.
Frequently Asked Questions
What does CMC stand for in pharmaceuticals?
CMC stands for Chemistry, Manufacturing, and Controls.
Why is CMC significant in drug development?
CMC is significant because it ensures that final pharmaceutical products are safe, effective, and compliant with regulatory requirements, which is crucial for their quality throughout the lifecycle.
What activities are included in CMC?
CMC encompasses the development of medication formulations, manufacturing processes, and the controls necessary to ensure that products meet rigorous quality standards.
What impact does inadequate CMC documentation have on drug approval?
Inadequate or incomplete CMC documentation can lead to substantial delays in approval timelines or even outright rejections of drug submissions.
How does CMC relate to Investigational New Drug (IND) submissions?
Over 40% of unsuccessful IND submissions for oncology therapies are due to challenges associated with CMC, highlighting the critical need for thorough CMC documentation and management.
What role does CMC play after a drug is approved?
CMC is essential for maintaining standards post-approval, ensuring ongoing compliance with regulatory requirements.
How is the complexity of pharmaceutical development affecting CMC?
As pharmaceutical development becomes more complex, the role of CMC in ensuring consistency and reliability in manufacturing processes becomes increasingly crucial.
What services does AVS Life Sciences provide related to CMC?
AVS Life Sciences provides management and regulatory compliance solutions, including GMP audits, to help navigate the complexities associated with CMC considerations and ensure adherence to regulatory standards.
