Understanding the CAPA Program: Importance and Key Components

Overview
The CAPA (Corrective and Preventive Action) program serves as a cornerstone in the pharmaceutical and biotechnology sectors, systematically identifying and resolving non-conformities while implementing preventive measures. This approach not only enhances overall quality and compliance but also addresses significant compliance challenges faced by the industry.
A well-structured CAPA program fulfills regulatory requirements and fosters a culture of continuous improvement, which is essential for ensuring patient safety and achieving operational excellence in a highly regulated environment.
By adopting such a program, organizations can navigate the complexities of compliance, demonstrating their commitment to quality and safety.
Introduction
The Corrective and Preventive Action (CAPA) program is a cornerstone in the pharmaceutical and biotechnology sectors, addressing the critical need for quality assurance and regulatory compliance. By systematically identifying and rectifying non-conformities, organizations enhance operational efficiency while safeguarding patient safety and product integrity.
However, with increasing regulatory scrutiny and the complexity of modern manufacturing processes, companies face significant compliance challenges. How can they ensure their CAPA initiatives are both effective and compliant?
This article delves into the significance of CAPA programs, exploring their essential components and the evolving landscape that shapes their implementation in the industry. Engage with us to discover actionable insights and compliance solutions that can elevate your CAPA initiatives.
Define CAPA Program: Key Concepts and Importance
The Corrective and Preventive Action program represents a structured methodology within the pharmaceutical and biotechnology sectors, designed to identify, investigate, and rectify non-conformities and potential issues in processes, products, or systems. Its primary objective extends beyond merely correcting existing problems; it also focuses on implementing measures to prevent their recurrence, thereby significantly enhancing overall quality and compliance. This corrective and preventive action process is integral to maintaining adherence to Good Manufacturing Practices (GMP), GXP, and other regulatory standards, as it empowers organizations to mitigate risks and enhance operational efficiency through effective documentation practices and internal auditing techniques.
Establishing an effective CAPA program fosters a culture of ongoing enhancement and responsibility, which is crucial in the highly regulated life sciences industry. Firms that have successfully integrated corrective and preventive actions into their management systems report improved compliance results and a reduction in occurrences of non-conformance. This proactive strategy not only addresses urgent problems but also contributes to achieving , ensuring that organizations remain competitive and compliant in a dynamic oversight environment.
Moreover, the execution of corrective and preventive actions is recognized as an essential component of a robust Quality Management System (QMS), with research indicating that efficient management of these actions can lead to a significant decrease in defects and reworks, ultimately enhancing patient safety and product standards. By prioritizing corrective and preventive actions, organizations can navigate the complexities of regulatory adherence while promoting innovation and efficiency in their operations. AVS Life Sciences stands as a reliable partner in delivering comprehensive management and compliance solutions, ensuring that organizations not only meet but exceed industry standards.
Contextualize CAPA in Pharmaceutical Compliance: Regulatory Framework and Industry Needs
Corrective and preventive action programs serve as crucial elements within the regulatory framework governing the pharmaceutical industry, particularly under the guidelines set forth by the FDA and ISO. These regulations mandate that organizations establish robust management systems, placing corrective and preventive actions at their core. The necessity for such actions stems from the industry's unwavering commitment to patient safety and product efficacy. Regulatory bodies expect firms to proactively identify and rectify quality issues, underscoring the pivotal role corrective and preventive actions play in this endeavor. Notably, the FDA has reported that four out of five warning letters issued are due to inadequate implementation and documentation of the corrective and preventive action process, highlighting the critical need for effective CAPA programs in this area.
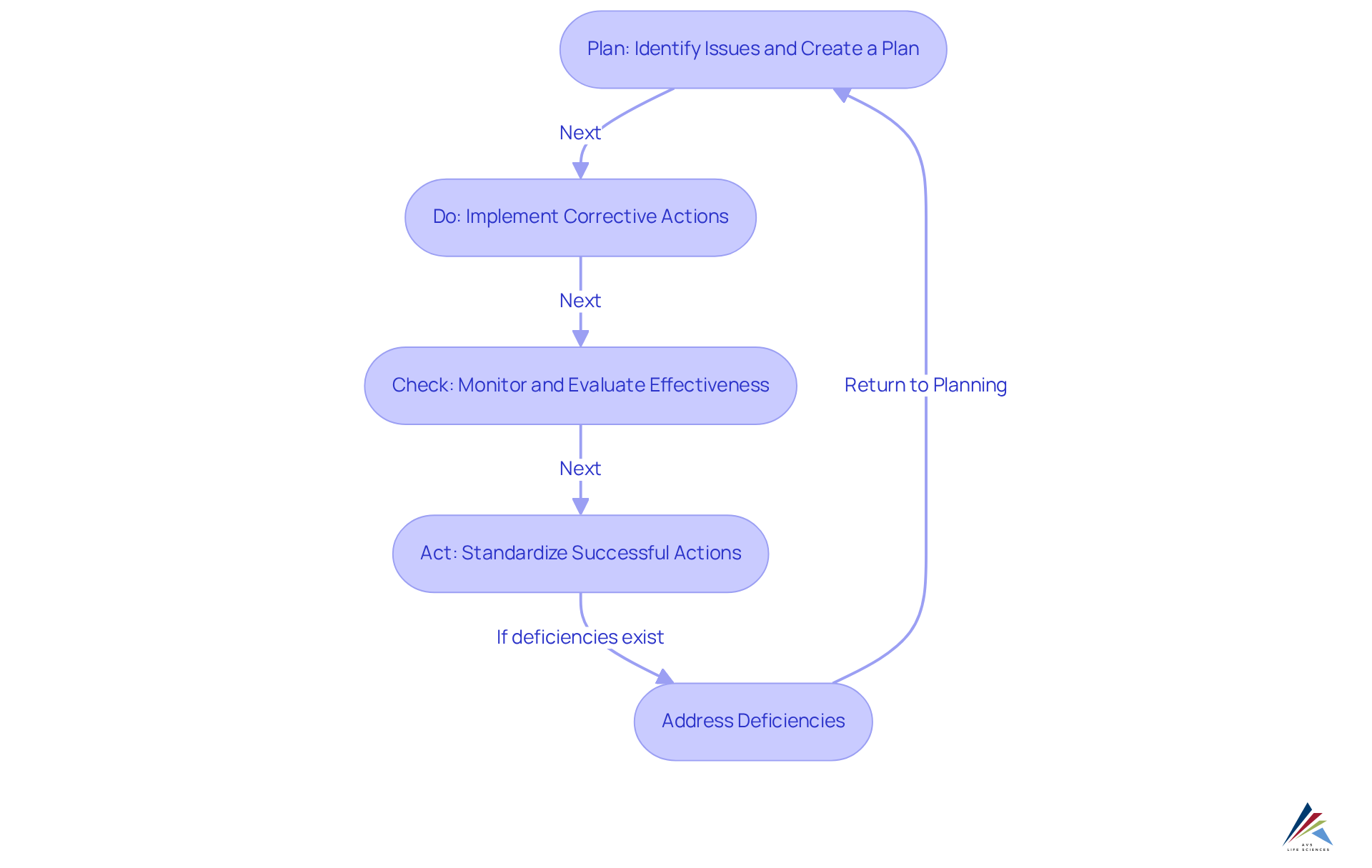
Moreover, the FDA's recent guidance emphasizes the importance of a systematic approach to corrective actions, advocating for the Plan-Do-Check-Act (PDCA) methodology to ensure compliance and effectiveness. Data from 2020 indicates that documentation deficiencies related to corrective and preventive action activities were widespread, signaling a significant opportunity for improvement within organizations. By adhering to these guidelines, companies not only protect public health but also enhance their reputation and operational integrity in a competitive landscape.
Real-world instances illustrate the impact of corrective and preventive actions on compliance and patient safety. For instance, Sun Pharmaceutical Industries Limited received a warning letter from the FDA due to inadequate cleaning and maintenance of manufacturing equipment, necessitating a comprehensive corrective and preventive action plan as part of the CAPA program to address identified deficiencies. This scenario underscores the importance of a that integrates thorough technical and scientific investigations.
Expert opinions further bolster the system's significance in FDA compliance, with industry leaders advocating for proactive measures as strategic investments that foster transparency and continuous improvement. In summary, effective corrective and preventive action initiatives transcend mere compliance obligations; they are vital for maintaining high standards of excellence and safety within the pharmaceutical sector.
Trace the Evolution of CAPA Programs: Historical Development and Milestones
The evolution of the CAPA program has been marked by significant milestones, reflecting the increasing complexity of pharmaceutical manufacturing and a heightened focus on quality assurance. Initially, corrective action systems were predominantly reactive, addressing issues only after they arose. However, as oversight expectations intensified—particularly with the introduction of the FDA's Quality System Regulation (QSR) in the 1990s—the emphasis shifted towards a proactive approach prioritizing prevention. This regulatory framework mandated the integration of the CAPA program into comprehensive management systems, laying the foundation for continuous improvement.
A transformative case study exemplifying this evolution is AVS Life Sciences' collaboration with a leading biotechnology company in San Francisco. AVS played a crucial role in upgrading their production area from a Biosafety Level 1 GMP site to a Level 2 GMP site, ensuring compliance with stringent assurance standards. The project was completed on time and within budget, underscoring AVS's commitment to enhancing control systems. The documentation efforts, which ensured complete traceability, received commendation from the client’s assurance team, highlighting the importance of robust corrective and preventive action practices in maintaining industry standards.
Throughout the project, critical lessons emerged, particularly regarding the barcode scanner issue, where some cameras were installed upside down, resulting in anomalies in test results that were initially recorded as “Passed.” This oversight underscored the necessity for thorough testing protocols and effective team communication concerning responsibilities. The experience prompted the Quality Control (QC) laboratory team and the Quality team to reevaluate their business processes, encouraging open discussions about workload and accountability.
As the sector has evolved, advancements in technology and data analysis have played a pivotal role in enhancing corrective and preventive action processes. Organizations now utilize sophisticated tools to analyze trends and identify potential issues before they escalate, thereby improving their ability to maintain compliance and enhance product quality. Statistics reveal that over half of all Form 483 observations and FDA warning letters cite deficiencies in corrective and preventive action systems, emphasizing the critical nature of effective CAPA program practices.
Key milestones in the development of the CAPA program include:
- The establishment of best practice guidelines recommending the closure of these actions within 60 days.
- Achieving a recurrence prevention success rate of at least 90%.
- The typical resolution duration from corrective action initiation to completion aims for 30 days, establishing a benchmark for efficiency.
- The target initial time through rate for corrective and preventive actions is greater than 99%, highlighting the effectiveness of these processes.
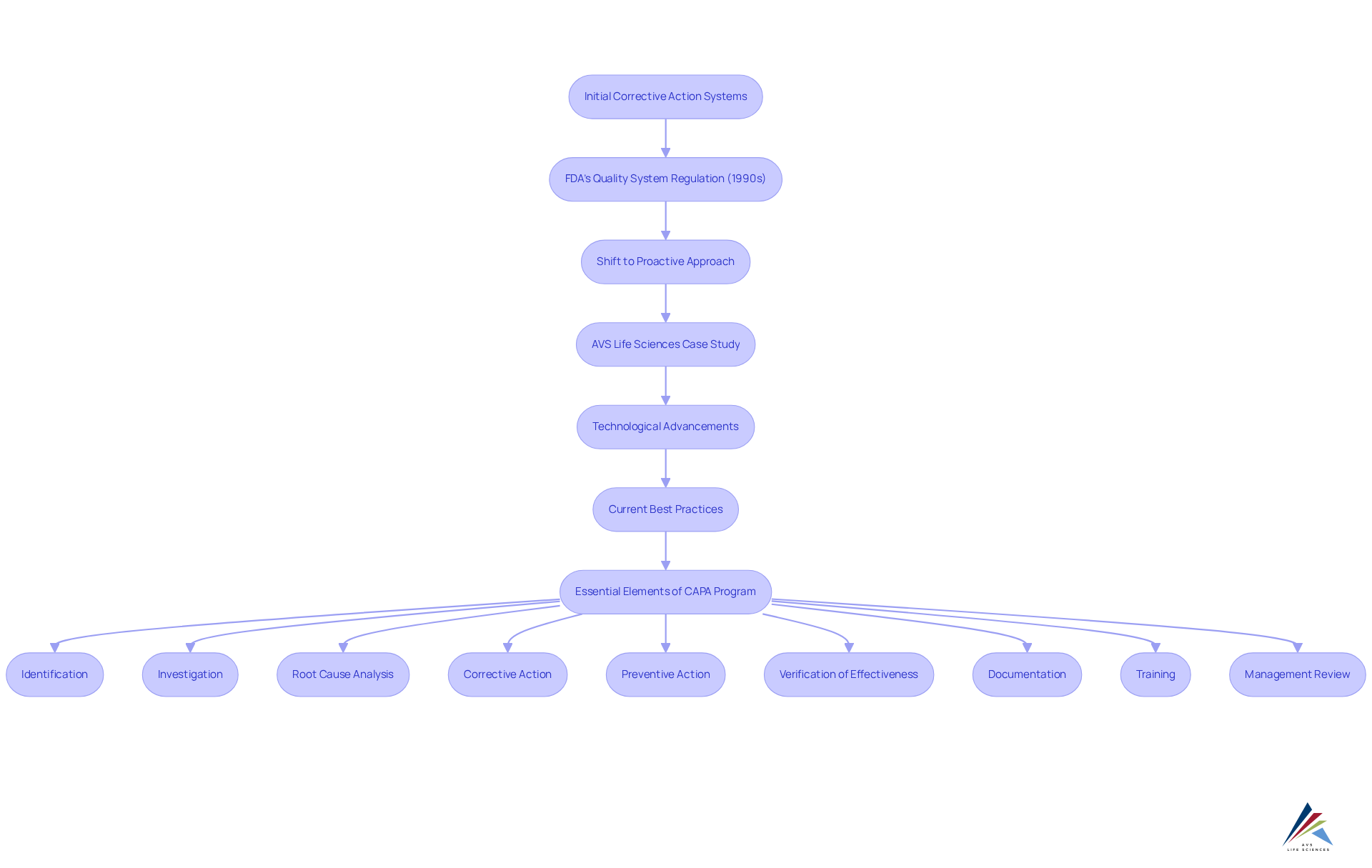
Monitoring corrective and preventive action timelines is crucial, particularly for those unresolved for over 90 days, as this indicates an issue requiring resolution. Additionally, a robust corrective and preventive action (CAPA program) must encompass nine essential elements:
- Identification
- Investigation
- Root cause analysis
- Corrective action
- Preventive action
- Verification of effectiveness
- Documentation
- Training
- Management review
This ensures comprehensive management of corrective measures.
The ongoing are vital as new standards of concern emerge, ensuring that organizations remain compliant and adaptable to oversight requirements. AVS Life Sciences' successful enhancement of the GMP facility not only exemplifies the practical application of these principles but also reinforces their commitment to comprehensive management and adherence solutions within the life sciences sector.
Outline Key Components of CAPA Programs: Structure and Processes
A well-organized capa program includes several essential elements that ensure effective oversight of non-conformities and compliance with regulatory standards. These components include:
- Identification: Recognizing non-conformities through various channels such as audits, customer complaints, and internal reviews is crucial. A robust nonconformance process can pinpoint issues before products reach the market.
- Investigation: Conducting thorough investigations to determine the root causes of identified issues is vital. Effective root cause analysis often necessitates time and cross-functional collaboration, employing methodologies like the 'Five Whys' to trace problems back to their origins.
- Action Plan: Developing a corrective action plan that delineates specific steps to address the root cause and prevent recurrence is essential. This plan should be targeted, evidence-based, and measurable, ensuring that corrective actions are verified prior to permanent implementation.
- Implementation: Executing the action plan involves informing and training all stakeholders as necessary. Proper training ensures that employees understand how to recognize and report quality issues, fostering a culture of accountability.
- Verification: Assessing the effectiveness of the corrective actions taken is crucial to confirm that issues have been resolved. Regular evaluations of effectiveness, including trend analysis and internal audits, reinforce a robust CAPA process and .
- Documentation: Maintaining detailed records of all corrective and preventive actions is vital for compliance with standards and continuous improvement. Comprehensive documentation fosters transparency and accountability, enabling organizations to monitor the effectiveness of their CAPA initiatives.
By adhering to these components, organizations can establish a robust capa program that addresses current issues while cultivating a culture of quality and compliance, ultimately enhancing operational efficiency and regulatory adherence.

Conclusion
The Corrective and Preventive Action (CAPA) program serves as a cornerstone framework within the pharmaceutical and biotechnology sectors, emphasizing the dual objectives of resolving existing issues and preventing future occurrences. This structured approach is essential for organizations striving to maintain compliance with regulatory standards while ensuring the highest quality of products and services. By embedding CAPA processes into their operational strategies, companies cultivate a culture of continuous improvement that ultimately enhances overall performance and competitive advantage.
Key insights from the discussion underscore the critical role of a robust CAPA program in fulfilling regulatory requirements established by bodies such as the FDA and ISO. Organizations that prioritize effective corrective and preventive actions can significantly reduce non-conformities, enhance patient safety, and improve compliance outcomes. The evolution of CAPA programs illustrates a shift from reactive measures to proactive strategies, highlighting the necessity for thorough investigations, strategic planning, and systematic implementation of corrective actions. Furthermore, the integration of advanced technology and data analysis has refined these processes, enabling better identification of potential issues before they escalate.
In light of these considerations, it is imperative for organizations in the life sciences industry to not only establish but also continuously refine their CAPA programs. By doing so, they ensure compliance with regulatory standards while contributing to a safer healthcare environment. Embracing the principles of effective CAPA management can lead to substantial improvements in quality assurance, operational efficiency, and ultimately, patient trust. The commitment to ongoing enhancement and accountability within CAPA programs transcends regulatory obligation; it represents a strategic investment in the future of pharmaceutical excellence.
Frequently Asked Questions
What is a CAPA program?
A CAPA program, or Corrective and Preventive Action program, is a structured methodology used in the pharmaceutical and biotechnology sectors to identify, investigate, and rectify non-conformities and potential issues in processes, products, or systems.
What are the main objectives of a CAPA program?
The primary objectives of a CAPA program are to correct existing problems and implement measures to prevent their recurrence, thereby enhancing overall quality and compliance.
Why is a CAPA program important in the life sciences industry?
A CAPA program is crucial in the life sciences industry as it helps maintain adherence to Good Manufacturing Practices (GMP), GXP, and other regulatory standards, mitigates risks, and enhances operational efficiency through effective documentation and internal auditing.
How does a CAPA program contribute to organizational culture?
Establishing an effective CAPA program fosters a culture of ongoing enhancement and responsibility, which is essential for organizations operating in highly regulated environments.
What benefits do firms experience by integrating CAPA actions into their management systems?
Firms that successfully integrate CAPA actions report improved compliance results and a reduction in occurrences of non-conformance, contributing to long-term operational excellence.
How does a CAPA program relate to Quality Management Systems (QMS)?
The execution of corrective and preventive actions is a vital component of a robust Quality Management System (QMS), and effective management of these actions can lead to a decrease in defects and reworks, enhancing patient safety and product standards.
What role does AVS Life Sciences play in CAPA management?
AVS Life Sciences provides comprehensive management and compliance solutions, ensuring that organizations not only meet but exceed industry standards in their CAPA programs.
