Understanding Quality Culture in the Pharmaceutical Industry

Overview
Quality culture in the pharmaceutical industry encompasses the collective values, beliefs, and actions that prioritize excellence across all organizational activities. This commitment ensures that products are safe, effective, and compliant with regulatory standards. A robust quality culture not only fosters accountability but also encourages continuous improvement among all employees. This leads to enhanced product standards, operational efficiency, and improved regulatory compliance, ultimately safeguarding patient well-being. By understanding and implementing these principles, organizations can navigate compliance challenges effectively and elevate their commitment to quality.
Introduction
Quality culture serves as the backbone of the pharmaceutical industry, shaping the standards and practices that ensure patient safety and product efficacy. By fostering an environment where excellence is prioritized, organizations can significantly enhance compliance with regulatory requirements and improve operational efficiency.
However, as the industry evolves, a pressing challenge emerges: how can companies maintain a robust quality culture amidst growing complexities and demands? This article delves into the essential characteristics of quality culture, its historical evolution, and its profound impact on pharmaceutical practices. It offers insights into how organizations can navigate this critical aspect of their operations, ultimately driving better outcomes and ensuring adherence to compliance standards.
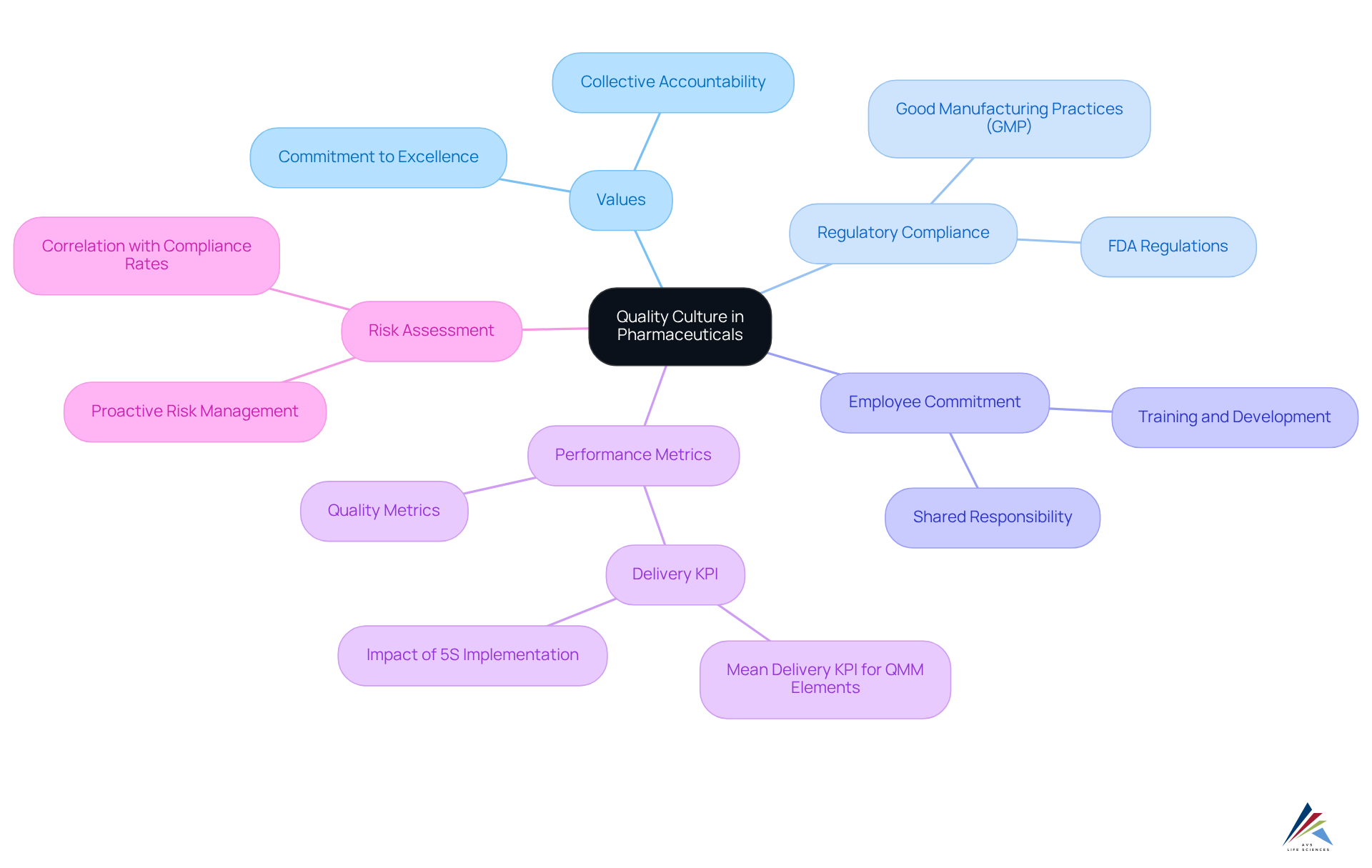
Define Quality Culture in the Pharmaceutical Context
The standard of excellence in the embodies the collective values, beliefs, and actions that prioritize excellence across all organizational activities. This environment is crucial for ensuring that products are safe, effective, and compliant with (GMP), GXP, FDA regulations, and other .
A robust fosters a shared commitment among all employees—from leadership to frontline workers—to uphold high standards and compliance. This collective accountability cultivates a quality culture in which standards are viewed as an integral aspect of every role, rather than solely the responsibility of the quality assurance team.
For instance, organizations that have successfully implemented excellence oversight practices report significantly improved , with establishments demonstrating advanced oversight practices achieving an average Delivery KPI of 54.7, compared to 42.0 for those with less developed practices.
Furthermore, the integration of has shown a positive correlation with , underscoring the importance of a proactive approach in mitigating risks and enhancing . As highlighted by industry experts, a well-established standard environment not only ensures reliable supply and fewer defects but also leads to efficiency gains, such as increased speed and throughput.
This comprehensive approach to excellence oversight, which includes adherence to , is vital for navigating the complexities of the pharmaceutical landscape and safeguarding patient well-being.
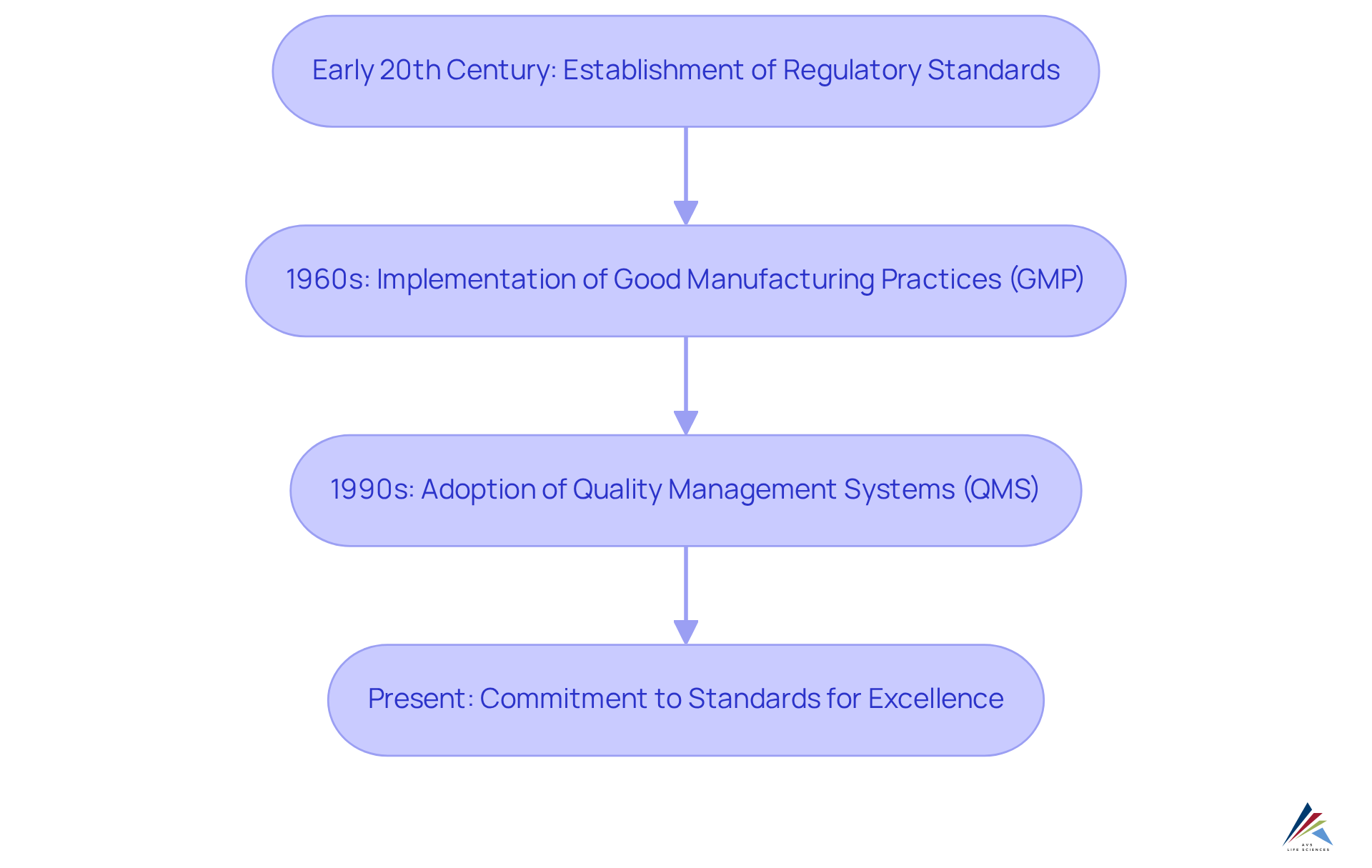
Trace the Evolution of Quality Culture in Pharmaceuticals
The development of excellence practices in the pharmaceutical sector commenced in the early 20th century, aligning with the establishment of aimed at ensuring drug safety and effectiveness. A pivotal moment occurred with the implementation of regulations in the 1960s, underscoring the necessity for systematic oversight. This transformation marked a transition from a compliance-oriented mentality to one that actively integrates excellence within the organizational environment.
By the 1990s, the advent of further solidified the , advocating for a holistic perspective that embraces regulatory compliance, continuous improvement, and employee engagement. Today, a robust is deemed essential for , with organizations recognizing that steadfast adherence to standards not only meets regulatory requirements but also fosters innovation and enhances overall performance.
A noteworthy illustration of this evolution is with a leading biotechnology company in San Francisco, where they successfully upgraded the client's manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project not only adhered to regulatory standards but also highlighted AVS's commitment to enhancing assurance processes.
Through this collaboration, valuable lessons emerged regarding the significance of thorough testing and the necessity for clear communication within teams to avert oversights. Case studies vividly demonstrate the profound on shaping excellence practices, revealing that organizations with a strong are better equipped to navigate the complexities of the pharmaceutical landscape.
Identify Key Characteristics of Quality Culture
Key characteristics of a strong in the pharmaceutical industry include:
- Leadership Commitment: Senior management must visibly support and prioritize improvement initiatives, demonstrating that excellence is a core value of the organization. This commitment is essential; organizations with engaged leadership are able to cultivate a quality culture that results in a significant increase in employee trust and productivity. Employees in high-trust environments report 50% higher productivity. AVS Life Sciences demonstrated this during a recent project where they supported a biotechnology firm in enhancing their , highlighting and ensuring patient well-being through strict oversight.
- : All employees should be actively involved in improvement processes, with a clear understanding of their roles in upholding standards. Engaged employees play a vital role in fostering a quality culture by taking ownership of processes and ensuring compliance in regulated industries. Research indicates that companies with a quality culture, marked by elevated employee involvement levels, witness enhanced performance and efficiency, directly influencing product standards and security. The AVS Life Sciences case study emphasizes how their partnership resulted in a more involved workforce concentrated on positive results, ultimately improving .
- : An environment of transparency encourages employees to report issues without fear of retribution, fostering a proactive approach to problem-solving. Effective communication is crucial; timely updates and leadership involvement diminish silos and foster trust, which is essential for establishing a quality culture. The AVS Life Sciences case study demonstrates how open conversations regarding workload and responsibilities can result in .
- : Organizations should adopt a mindset of ongoing learning and adaptation, regularly evaluating and enhancing their practices. This dedication to ongoing enhancement not only boosts adherence but also , inspiring involved employees to share ideas and collaborate on improvement initiatives. The initiatives by AVS Life Sciences in pinpointing deficiencies throughout their project illustrate the importance of ongoing enhancement in quality management, resulting in improved protocols and product dependability.
- Patient Protection Emphasis: A quality ethos must , ensuring that all decisions and actions align with the goal of delivering secure and effective products. Leadership must model accountability and transparency in decision-making, reinforcing the importance of patient safety as a fundamental organizational value. By promoting a quality culture that emphasizes these traits, pharmaceutical firms can elevate their processes and ultimately enhance patient results.

Explain the Importance of Quality Culture in the Pharmaceutical Industry
is essential in the pharmaceutical industry for several compelling reasons:
- : A strong organizational culture promotes adherence to regulatory requirements, significantly minimizing the risk of non-compliance and the related penalties. Organizations that cultivate a quality culture are more likely to navigate complex regulatory landscapes effectively, ensuring they remain 'recall ready' as emphasized by the FDA. illustrates this dedication by helping clients enhance their facilities to satisfy strict , thus strengthening regulatory compliance and assurance of excellence.
- Improved Product Standard: Firms with a robust excellence environment consistently produce superior products, reducing flaws and withdrawals. Entities that have adopted thorough management systems indicate that 83% think these solutions have helped in recovering from , emphasizing the direct link between a quality culture and product integrity. Moreover, 94% of survey participants have a dedicated function that fosters a quality culture, highlighting the organizational dedication to standards.
- : By cultivating a quality culture of , companies can streamline processes, reduce waste, and enhance productivity. This operational efficiency not only aids in improved results but also facilitates a more agile reaction to market demands. AVS Life Sciences' participation in biotechnology facility enhancements demonstrates how efficient management leads to operational excellence, enabling clients to concentrate on creating life-saving medicines.
- Risk Mitigation: A proactive enables organizations to recognize and tackle potential problems before they intensify, thereby diminishing threats to patient well-being and safeguarding the organization's reputation. The increase in recalls linked to supply chain disruptions highlights the significance of having a strong assurance mechanism in place. AVS Life Sciences provides essential services that help clients implement effective , ensuring patient safety and product integrity.
- Companies that emphasize a quality culture of excellence are better positioned to meet market demands and build trust with stakeholders. This dedication to excellence boosts customer loyalty and propels business success, as shown by the growing emphasis on among organizations, with 42% reporting an increase in their investments for enhancement. Furthermore, 56% of organizations saw a rise in product recalls, highlighting the necessity for strong control systems and proactive monitoring to tackle possible safety concerns early.
In summary, the influence of quality culture on product standards and recall rates in the pharmaceutical sector is profound. Organizations that integrate a quality culture into their core values not only enhance their operational effectiveness but also protect public health and uphold regulatory compliance. AVS Life Sciences exemplifies this commitment through its successful projects, reinforcing that quality excellence is a continuous process requiring ongoing commitment from all levels of the organization.

Conclusion
A strong quality culture in the pharmaceutical industry transcends regulatory requirements; it is a cornerstone that drives excellence in product safety and efficacy. By fostering an environment where every employee, from leadership to frontline workers, is dedicated to high standards, organizations can embed quality in every aspect of their operations. This collective commitment not only enhances compliance but also nurtures an atmosphere where innovation and continuous improvement flourish.
Throughout this article, we have explored key elements of a robust quality culture, including the essential roles of:
- Leadership commitment
- Employee engagement
- Open communication
- A focus on patient protection
Historical insights illustrate the evolution of quality culture, showcasing how the pharmaceutical sector has shifted from mere compliance to embracing excellence as a core value. Case studies, such as those involving AVS Life Sciences, further exemplify the tangible benefits of a strong quality culture, including improved product standards, operational efficiency, and effective risk mitigation.
Ultimately, the significance of quality culture in the pharmaceutical industry cannot be overstated. Organizations that prioritize quality not only safeguard public health but also enhance their operational effectiveness and build trust with stakeholders. As the industry continues to confront evolving challenges, a steadfast commitment to quality culture will be essential for navigating complexities and achieving sustainable success. Embracing this culture is not merely a strategic advantage; it is a moral imperative that reinforces the industry's dedication to delivering safe and effective products.
Frequently Asked Questions
What is quality culture in the pharmaceutical context?
Quality culture in the pharmaceutical sector refers to the collective values, beliefs, and actions that prioritize excellence across all organizational activities, ensuring that products are safe, effective, and compliant with regulatory standards.
Why is a robust quality culture important?
A robust quality culture fosters shared commitment among all employees to uphold high standards and compliance, making quality an integral aspect of every role rather than just the responsibility of the quality assurance team.
How does quality culture impact delivery performance?
Organizations with successful excellence oversight practices report significantly improved delivery performance, with those demonstrating advanced oversight achieving an average Delivery KPI of 54.7, compared to 42.0 for those with less developed practices.
What is the role of risk assessment in quality culture?
The integration of risk assessment processes is positively correlated with compliance rates, highlighting the importance of a proactive approach in mitigating risks and enhancing product excellence.
What benefits does a well-established quality culture provide?
A well-established quality culture ensures reliable supply, fewer defects, and leads to efficiency gains, such as increased speed and throughput in operations.
How does adherence to standard operating procedures (SOPs) relate to quality culture?
Adherence to SOPs is vital for navigating the complexities of the pharmaceutical landscape and safeguarding patient well-being as part of a comprehensive approach to excellence oversight.
