Understanding QPPV: Key Role in Pharmaceutical Compliance

Overview
The article examines the pivotal role of the Qualified Person for Pharmacovigilance (QPPV) in upholding pharmaceutical compliance and ensuring drug safety. It underscores the QPPV's essential function in:
- Monitoring the safety of medicinal products
- Managing adverse event reporting
- Ensuring adherence to regulatory requirements
This multifaceted role is vital for maintaining public health standards, showcasing the QPPV's contribution to the integrity of the pharmaceutical industry.
Introduction
The role of the Qualified Person for Pharmacovigilance (QPPV) has become increasingly crucial in the pharmaceutical industry, serving as the linchpin in ensuring the safety and compliance of medicinal products. As regulatory scrutiny intensifies and the landscape of drug safety evolves, understanding the responsibilities and significance of the QPPV is essential for maintaining public trust and health.
With rapid advancements in technology and the growing complexity of pharmacovigilance, QPPVs face significant challenges in navigating these dynamics. How can they effectively safeguard patient safety and adhere to stringent compliance requirements?
This article will explore these pressing issues and provide actionable insights for compliance solutions that enhance the role of QPPVs in today’s complex environment.
Define QPPV: The Qualified Person for Pharmacovigilance
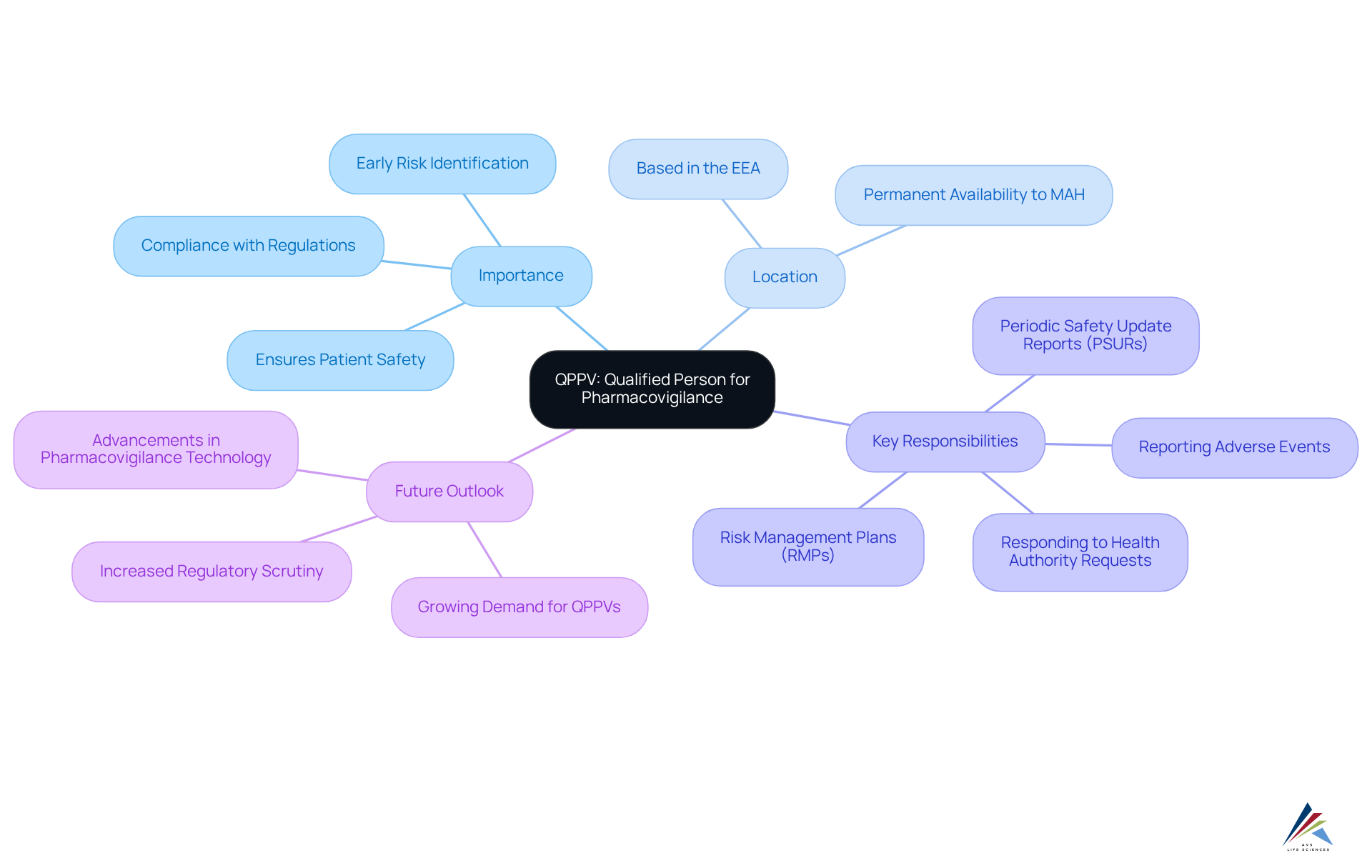
The QPPV, which stands for Qualified Person for Pharmacovigilance, is an indispensable figure within pharmaceutical companies, tasked with overseeing the pharmacovigilance system. This role is vital for the ongoing monitoring of the safety of medicinal products, ensuring that any adverse effects are reported in compliance with regulatory mandates. The individual designated as the QPPV must be based within the European Economic Area (EEA) and holds legal accountability for the safety of all marketed products, thus playing a pivotal role in maintaining public health and safety standards.
By 2025, the EEA is anticipated to feature a robust network of QPPVs, reflecting the growing demand for qualified professionals in this sector. Each pharmaceutical firm is required to designate at least one individual as the QPPV responsible for the comprehensive pharmacovigilance of all medicinal products for which they hold marketing authorizations. Key responsibilities of the QPPV encompass:
- The timely reporting of adverse events
- The development and maintenance of Risk Management Plans (RMPs)
- The preparation of Periodic Safety Update Reports (PSURs) to ensure compliance with regulatory requirements
The importance of QPPV in pharmaceutical compliance cannot be overstated. Their expertise is crucial in early identification of potential risks, facilitating timely interventions that can avert harm to patients. As a cornerstone of the pharmacovigilance system, the QPPV plays a crucial role in ensuring that companies adhere to Good Pharmacovigilance Practices (GVP), thereby protecting both public health and the integrity of the pharmaceutical industry.
Contextualize QPPV: Its Importance in Pharmacovigilance
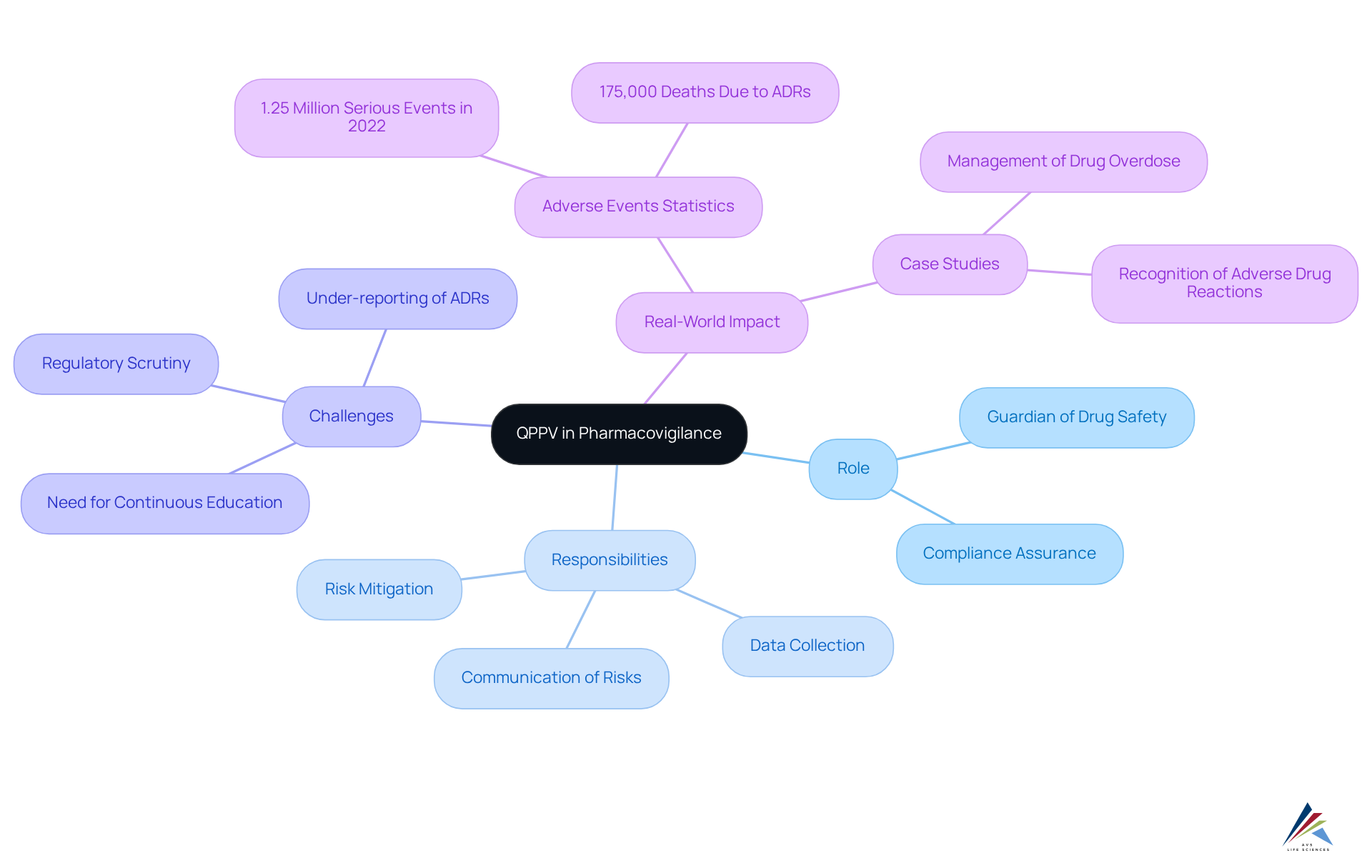
The Qualified Individual for Pharmacovigilance occupies a pivotal role in the field of pharmacovigilance, which focuses on monitoring the safety of medications and implementing QPPV strategies to mitigate risks while enhancing benefits. As pharmaceutical companies face increasing scrutiny from regulatory authorities, the Qualified Person under the QPPV framework ensures that all risk-related data is meticulously collected, analyzed, and communicated. This position transcends mere compliance; it embodies a commitment to patient safety and the preservation of trust within the healthcare system.
Acting as a guardian of drug safety, the QPPV is tasked with swiftly identifying and addressing any potential hazards associated with medications. In 2022 alone, over 1.25 million significant adverse events were reported in the U.S., underscoring the critical nature of this role in bolstering drug safety and compliance.
Real-world examples illustrate the profound impact of the QPPV in pharmacovigilance: through proactive monitoring and timely reporting, these professionals have effectively mitigated risks associated with high-profile medications, thereby reinforcing their essential function in maintaining public confidence in pharmaceutical products.
Outline Key Responsibilities of the QPPV in Drug Safety
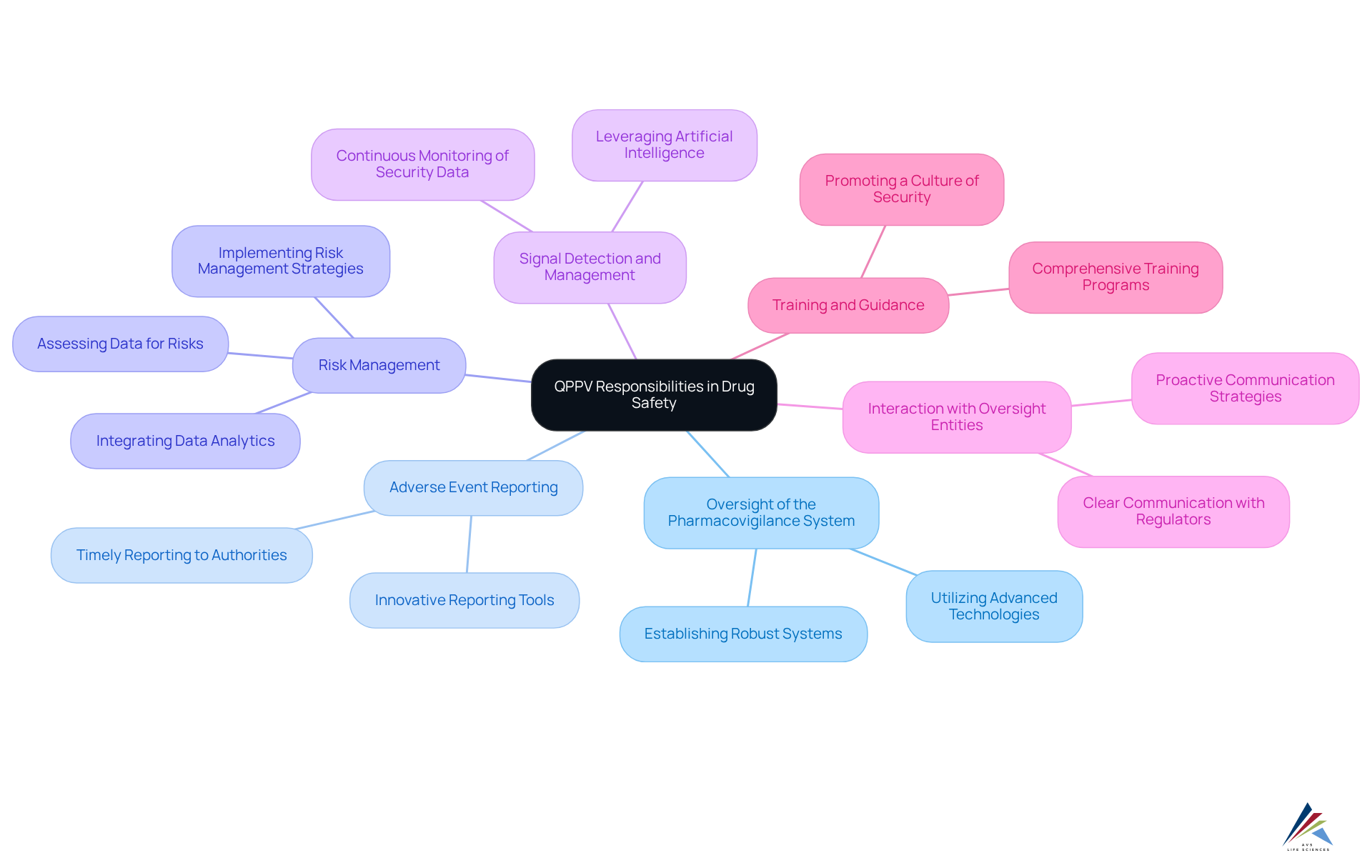
The responsibilities of the Qualified Person for Pharmacovigilance (QPPV) encompass several key areas essential for maintaining drug safety and regulatory compliance:
-
Oversight of the Pharmacovigilance System: The QPPV is tasked with establishing and maintaining a robust pharmacovigilance system that adheres to all applicable regulations, thereby ensuring comprehensive safety monitoring. AVS Life Sciences employs advanced technologies to enhance the efficiency and effectiveness of these systems.
-
Adverse Event Reporting: Timely reporting of all adverse drug reactions (ADRs) to the relevant regulatory authorities is a critical duty. This includes staying updated on regulations to ensure compliance with the latest reporting requirements. AVS Life Sciences utilizes innovative reporting tools to streamline this process and improve accuracy.
-
Risk Management: The qualified person responsible for pharmacovigilance assesses data regarding risks to identify potential hazards linked to pharmaceutical products, particularly focusing on risk management. They implement effective risk management strategies, which may include risk minimization plans and post-marketing surveillance to mitigate identified risks. AVS Life Sciences integrates data analytics to enhance risk assessment and management practices.
-
Signal Detection and Management: Continuous monitoring of security data is essential for identifying emerging concerns. The qualified person responsible for pharmacovigilance must handle these signals efficiently, ensuring that any required measures are implemented to safeguard patient well-being. AVS Life Sciences leverages artificial intelligence to improve signal detection capabilities.
-
Interaction with Oversight Entities: Serving as the main intermediary with oversight bodies, the QPPV ensures clear communication concerning pharmacovigilance issues, meeting all legal requirements and upholding adherence to oversight expectations. AVS Life Sciences emphasizes proactive communication strategies to foster strong relationships with regulators.
-
Training and Guidance: The QPPV plays a crucial role in promoting a culture of security within the organization by offering training and direction to staff on pharmacovigilance practices. This includes sharing best practices for adverse event reporting and risk management strategies, which are crucial for effective pharmacovigilance. AVS Life Sciences has developed comprehensive training programs that have shown to improve reporting rates and better risk management outcomes.
By following these duties, the qualified person for pharmacovigilance not only guarantees conformity with legal frameworks but also improves the overall protection of pharmaceutical products in the market, aligning with AVS Life Sciences' dedication to quality management and legal adherence.
The Evolving Role of the QPPV in Modern Pharmacovigilance
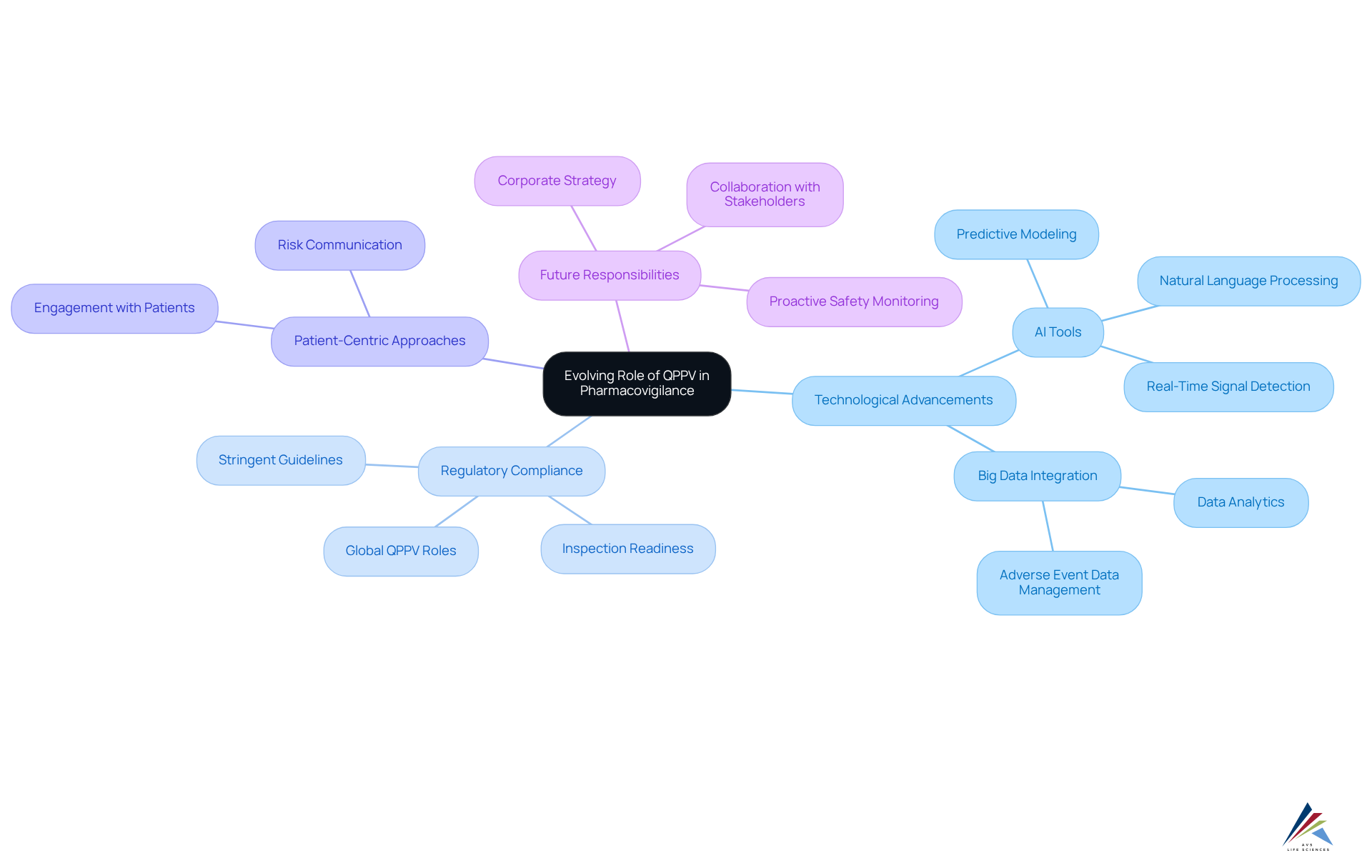
The function of the QPPV is rapidly evolving, driven by technological advancements, policy changes, and the complexities of modern drug development. With the integration of big data and artificial intelligence (AI), QPPVs are increasingly expected to utilize these tools for enhanced signal detection and risk management. AI-powered systems, for instance, can analyze extensive datasets, significantly enhancing the detection of adverse drug reactions (ADRs) and enabling proactive measures. The global pharmacovigilance market, estimated at approximately USD 8.5 billion, reflects the industry's response to the critical demand for improved drug safety protocols and regulatory compliance.
As regulatory authorities impose more stringent guidelines, QPPVs must remain vigilant and well-informed about these changes to ensure compliance with QPPV standards. The adoption of AI technologies, including predictive modeling and natural language processing, empowers QPPVs to optimize data analysis and enhance the accuracy of adverse event reporting. This technological transformation not only facilitates timely decision-making but also aligns with the increasing focus on patient-centric approaches in drug development. Consequently, QPPVs are expected to engage more actively with patients and healthcare professionals, thereby broadening their role within the pharmaceutical landscape.
Furthermore, the rising volume of adverse event data necessitates that QPPVs leverage advanced analytics to effectively manage and interpret this information, which is crucial for QPPV activities. By 2025, the responsibilities of the QPPV are anticipated to further evolve, with an enhanced emphasis on corporate strategy and risk communication tailored to patients and caregivers. Organizations that equip their QPPV with the necessary resources and authority are better positioned to achieve compliance, ensure inspection readiness, and build trust with regulators. Additionally, the integration of AI in project management can further bolster the efficiency and effectiveness of QPPV responsibilities, ensuring that safety measures are not merely reactive but also proactive in nature.
Conclusion
The Qualified Person for Pharmacovigilance (QPPV) plays an essential role in the pharmaceutical industry, ensuring meticulous monitoring of drug safety and adherence to regulatory compliance. This position underscores the critical nature of timely reporting and risk management, as well as the QPPV's accountability for the safety of marketed medicinal products. As the field of pharmacovigilance continues to evolve, the importance of the QPPV in safeguarding public health remains paramount.
This article outlines the multifaceted responsibilities of the QPPV, including:
- Oversight of pharmacovigilance systems
- Adverse event reporting
- Risk management
- Engagement with regulatory bodies
The incorporation of advanced technologies, such as artificial intelligence, is revolutionizing these responsibilities, enabling QPPVs to improve their effectiveness in identifying and mitigating risks associated with drug safety. The proactive stance adopted by QPPVs not only fosters compliance but also strengthens the trustworthiness of the pharmaceutical sector.
Given the increasing complexities in drug development and the escalating volume of adverse event data, organizations must prioritize empowering their QPPV roles. By investing in training, resources, and technological advancements, pharmaceutical companies can ensure their QPPVs are well-prepared to tackle the challenges ahead. Ultimately, a commitment to robust pharmacovigilance practices not only safeguards patient safety but also upholds the integrity of the healthcare system, rendering the role of the QPPV more critical than ever.
Frequently Asked Questions
What does QPPV stand for?
QPPV stands for Qualified Person for Pharmacovigilance.
What is the role of a QPPV?
The QPPV oversees the pharmacovigilance system within pharmaceutical companies, ensuring the ongoing monitoring of the safety of medicinal products and compliance with regulatory mandates regarding adverse effect reporting.
Where must the QPPV be located?
The QPPV must be based within the European Economic Area (EEA).
What are the legal responsibilities of a QPPV?
The QPPV holds legal accountability for the safety of all marketed products and is responsible for maintaining public health and safety standards.
What are the key responsibilities of a QPPV?
Key responsibilities include the timely reporting of adverse events, the development and maintenance of Risk Management Plans (RMPs), and the preparation of Periodic Safety Update Reports (PSURs) to ensure compliance with regulatory requirements.
Why is the QPPV important for pharmaceutical compliance?
The QPPV is crucial for the early identification of potential risks, facilitating timely interventions that can prevent harm to patients and ensuring adherence to Good Pharmacovigilance Practices (GVP).
What is the anticipated trend for QPPVs in the EEA by 2025?
By 2025, the EEA is expected to have a robust network of QPPVs due to the growing demand for qualified professionals in this sector.
