Understanding Pharmaceutical Regulatory Affairs and Its Importance

Overview
Pharmaceutical regulatory affairs play a pivotal role in ensuring that medicinal products meet stringent safety, efficacy, and quality standards throughout their development and market approval processes. Compliance specialists are integral in navigating these complex regulations, significantly impacting public health. Their expertise not only facilitates adherence to these standards but also prevents unsafe products from entering the market, thereby safeguarding consumers and maintaining the integrity of the pharmaceutical industry. By understanding and implementing effective compliance strategies, stakeholders can enhance their operational frameworks and contribute to a healthier society.
Introduction
The intricate world of pharmaceutical regulatory affairs plays a pivotal role in ensuring that medications are not only effective but also safe for public consumption. As the landscape of drug development becomes increasingly complex, the importance of compliance with regulatory standards has never been more pronounced. This article delves into the essential functions of regulatory affairs professionals, exploring how their expertise safeguards public health while navigating the evolving challenges of the pharmaceutical industry.
What happens when compliance fails, and how can organizations mitigate the risks associated with non-compliance? By addressing these questions, we highlight the critical need for robust compliance solutions and the proactive measures organizations can take to ensure adherence to regulatory requirements.
Define Pharmaceutical Regulatory Affairs and Its Role
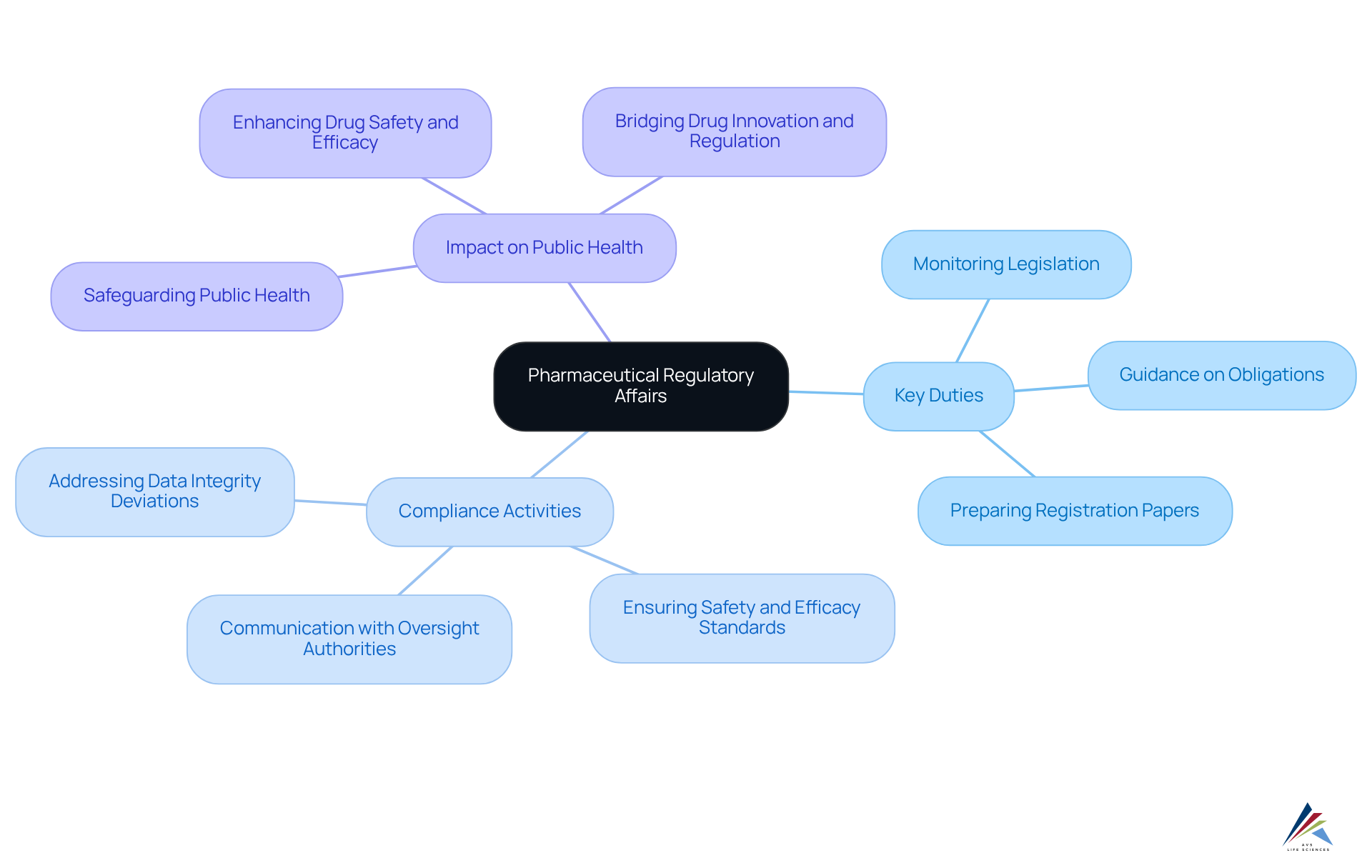
The field of pharmaceutical regulatory affairs represents a specialized discipline dedicated to ensuring that medicinal products adhere to established rules and guidelines. This area encompasses a variety of activities, including the preparation and submission of compliance documents, effective communication with oversight authorities, and the assurance that products meet stringent safety, efficacy, and quality standards. Compliance specialists serve as crucial intermediaries in pharmaceutical regulatory affairs between drug manufacturers and oversight organizations, ensuring adherence to legal and ethical standards throughout the medication development process—from initial research to market approval. Their work is vital in and upholding the integrity of the pharmaceutical industry.
As we look to 2025, the significance of compliance matters is underscored by the growing complexity of drug development and the evolving oversight environment. Experts in this domain are tasked with overcoming these challenges, ensuring adherence to Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR). They address critical aspects such as Data Integrity Deviations, Investigations, and CAPA, all essential for maintaining compliance and avoiding pitfalls. Their strategic involvement not only assists companies in navigating issues related to inadequate record-keeping and data presentation but also significantly impacts compliance decisions, thereby enhancing drug safety and efficacy statistics.
Key duties of compliance specialists in the field of pharmaceutical regulatory affairs include:
- Monitoring evolving legislation
- Providing guidance on legal and scientific obligations
- Preparing registration papers for submission to oversight agencies
Their expertise is crucial in negotiating marketing authorizations and ensuring that products are both safe and beneficial to public health. As one expert in regulation noted, their role is pivotal in bridging the gap between drug innovation and adherence to regulations, ultimately contributing to the commercial and scientific success of development initiatives. Organizations such as AVS Life Sciences are committed to delivering comprehensive quality management and compliance solutions, enhancing the capabilities and standing of compliance professionals in the sector. This dedication to excellence positions compliance management as a foundational element of the drug industry.
Trace the History and Evolution of Regulatory Affairs
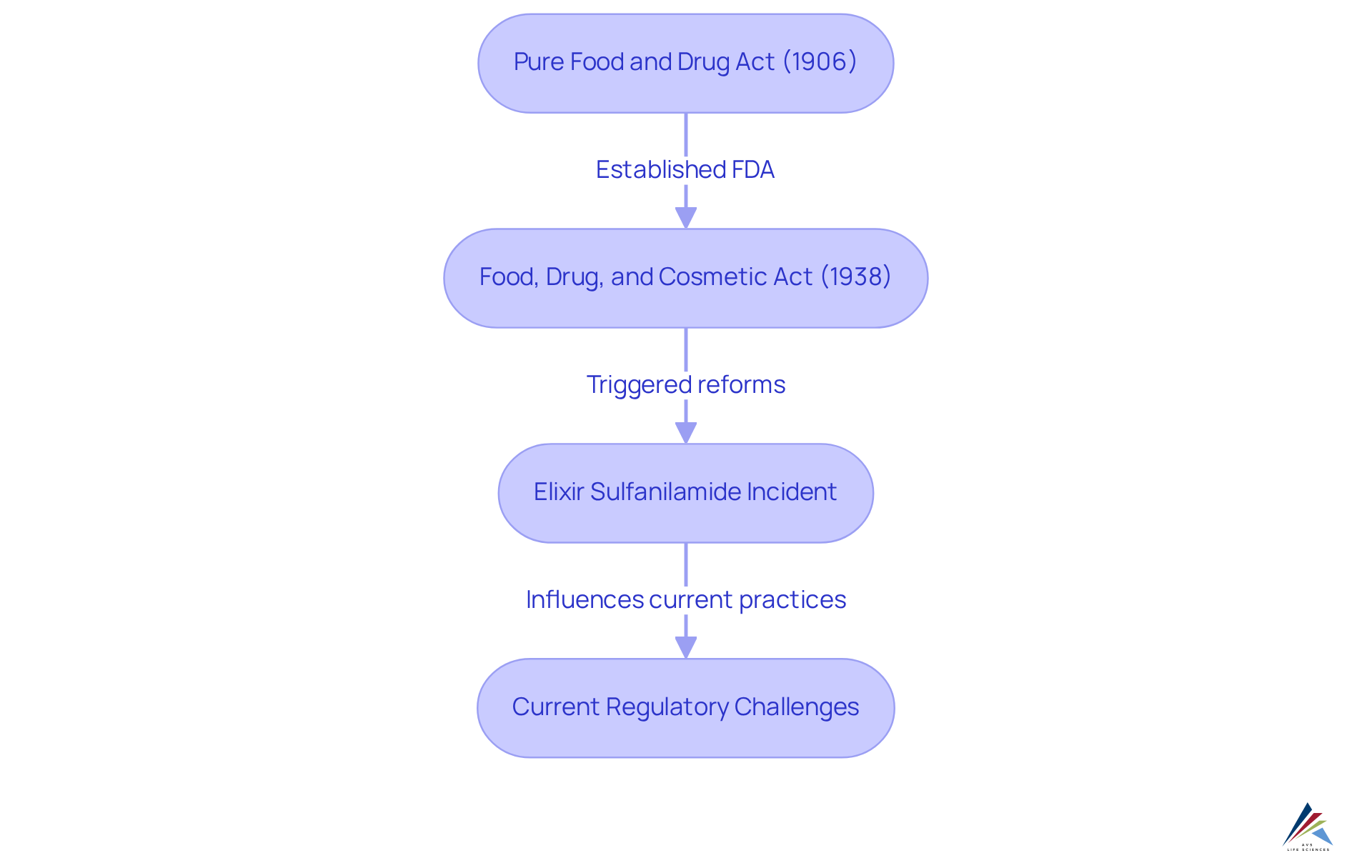
The history of drug oversight traces back to the early 20th century, characterized by pivotal legislation such as the Pure Food and Drug Act of 1906. This landmark act aimed to eradicate the sale of misbranded or adulterated foods and drugs, laying the foundation for modern consumer protection. Subsequently, the Food, Drug, and Cosmetic Act of 1938 further consolidated the FDA's authority to oversee drug safety, instituting rigorous standards for product approval and monitoring.
Over the decades, the evolution of compliance processes in pharmaceutical regulatory affairs has been driven by numerous public health crises, technological advancements, and the increasing globalization of the pharmaceutical industry. For example, the FDCA was significantly shaped by a mass poisoning incident involving elixir sulfanilamide, which resulted in over 100 fatalities and underscored the pressing need for stringent regulations. This event catalyzed substantial reforms that bolstered consumer safety and curtailed the prevalence of quack medicines.
Case studies underscore the profound impact of the Pure Food and Drug Act. Investigative journalism, particularly by muckrakers like Samuel Hopkins Adams, was instrumental in elevating public awareness about the perils of patent medicines, ultimately facilitating the act's passage. Moreover, the experiments conducted by Dr. Harvey Washington Wiley, known as the 'Poison Squad,' highlighted the harmful effects of food preservatives, influencing public sentiment and regulatory practices.
Today, compliance affairs professionals, including those at AVS Life Sciences, face the challenge of navigating a complex landscape of international regulations, which underscores the importance of for achieving harmonization in a globalized industry. AVS Life Sciences leverages historical insights to inform current practices, ensuring that quality management remains paramount in drug development. As the pharmaceutical sector continues to evolve, the lessons gleaned from past pharmaceutical regulatory affairs frameworks are vital in safeguarding drug safety and effectiveness.
Identify Key Components and Responsibilities in Regulatory Affairs
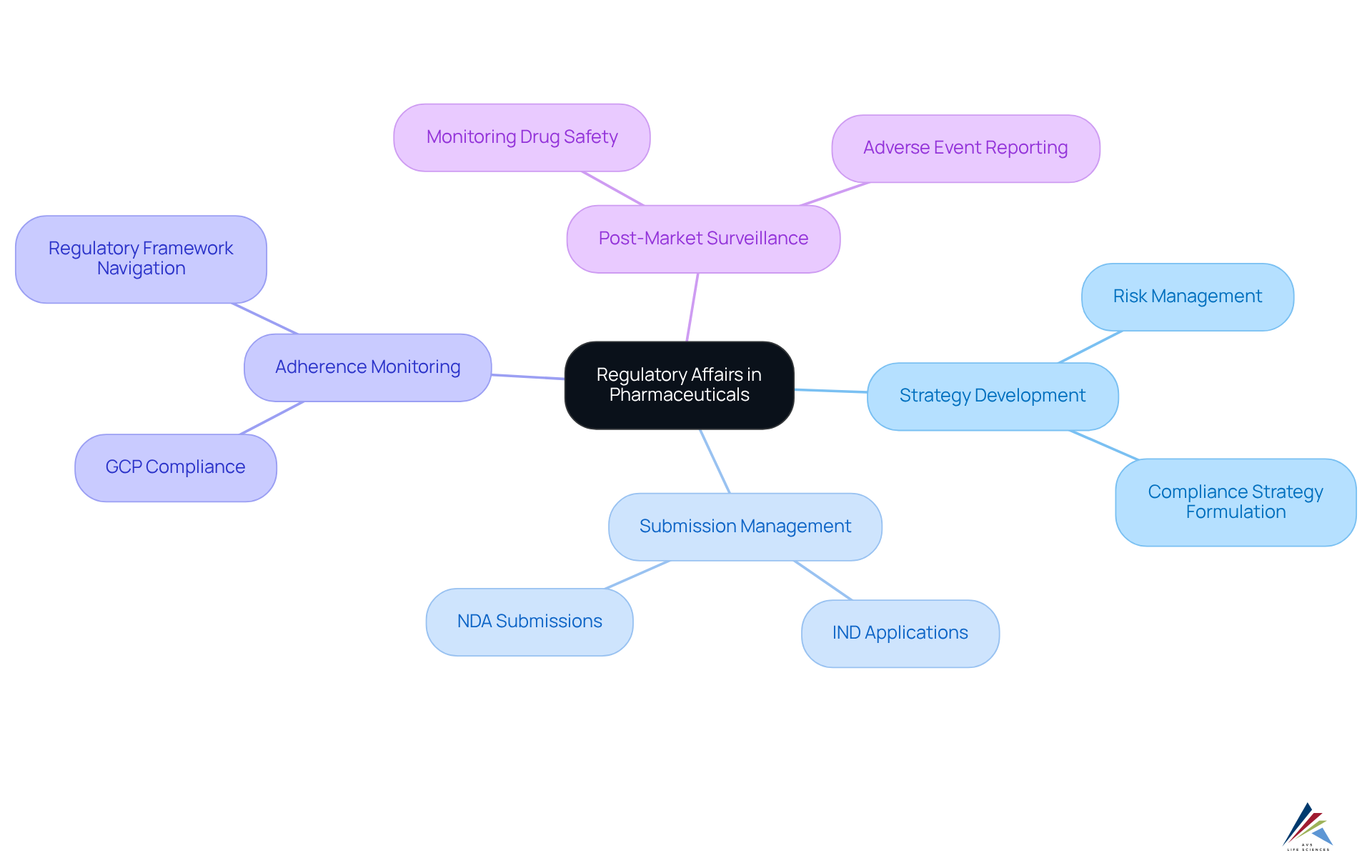
Oversight affairs in pharmaceutical regulatory affairs encompass several critical elements, including strategy development, submission management, adherence monitoring, and post-market surveillance. Professionals in pharmaceutical regulatory affairs are responsible for preparing and submitting essential documentation to oversight agencies, including Investigational New Drug (IND) applications and New Drug Applications (NDA). They ensure that [clinical trials comply with Good Clinical Practice (GCP) guidelines](https://avslifesciences.com/medical-devices-invitro-diagnostics) and maintain adherence to pharmaceutical regulatory affairs throughout the product lifecycle.
A pivotal aspect of their responsibilities in pharmaceutical regulatory affairs involves managing risks, where they assess potential compliance risks and formulate strategies to mitigate them. This proactive approach is vital, especially considering that the composite success rate for clinical development rose to 10.8% in 2023. This statistic underscores the importance of in achieving successful outcomes.
Compliance affairs specialists also collaborate with cross-functional teams, including research and development, quality assurance, and marketing, to ensure that all aspects of product development adhere to pharmaceutical regulatory affairs. Their ability to navigate complex oversight frameworks in pharmaceutical regulatory affairs is crucial for minimizing delays and enhancing submission efficiency, particularly as governing bodies impose increasingly stringent standards. Organizations that prioritize the development of compliance strategies in pharmaceutical regulatory affairs are better positioned to manage submissions effectively, ensuring their products meet the necessary approval criteria.
At AVS Life Sciences, we provide tailored consulting solutions in pharmaceutical regulatory affairs and compliance, assisting organizations in navigating these complexities. Our expertise in pharmaceutical regulatory affairs simplifies the compliance process, ensuring that your submissions are both compliant and strategically aligned with industry standards.
Highlight the Importance of Regulatory Affairs in Ensuring Compliance and Safety
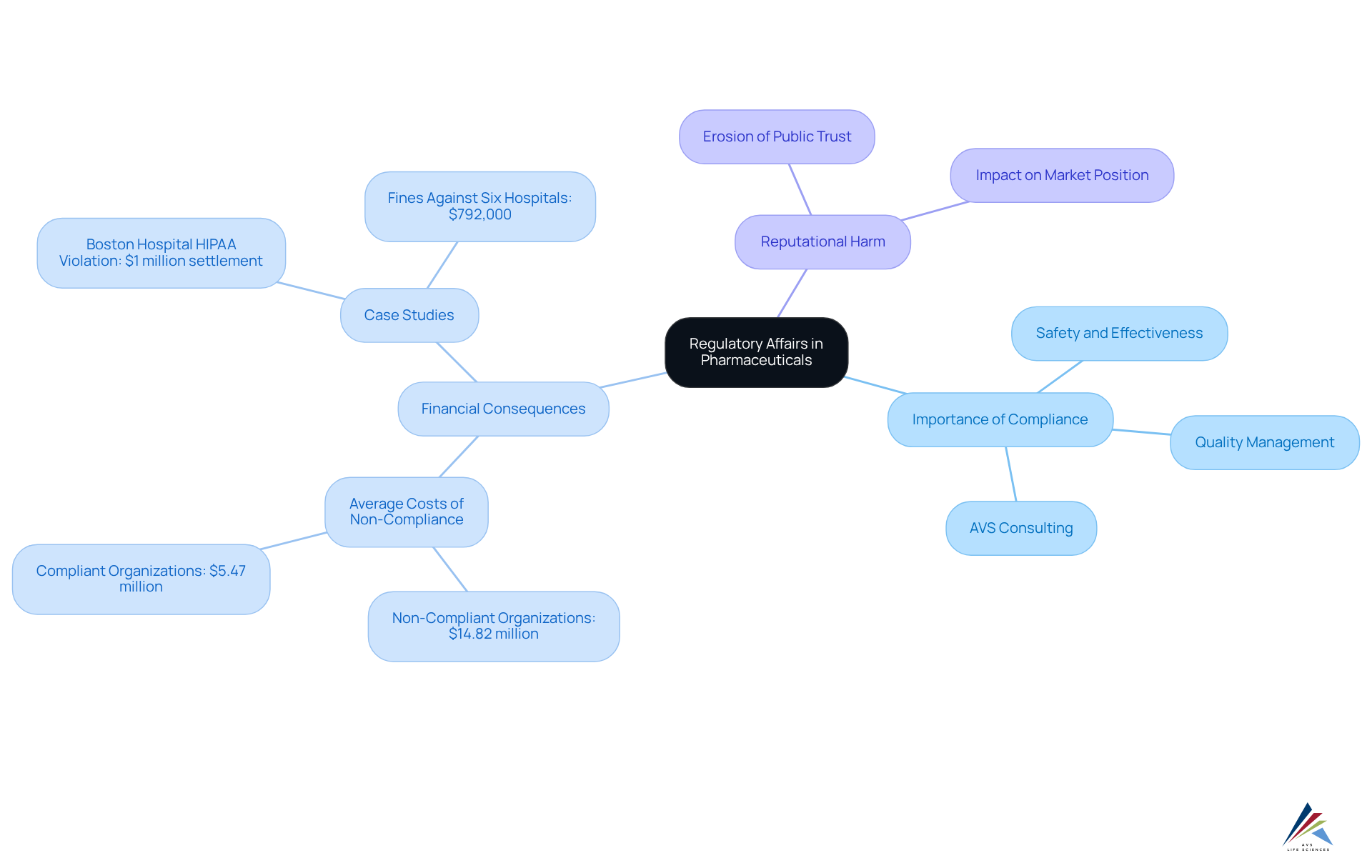
Pharmaceutical regulatory affairs are crucial in ensuring that pharmaceutical products are safe, effective, and compliant with applicable laws and regulations. At AVS Life Sciences, we provide extensive quality management and adherence solutions tailored for the life sciences sector. The rigorous procedures involved in compliance submissions and approvals aim to prevent unsafe or ineffective products from entering the market. Non-compliance can lead to severe consequences, including product recalls, legal penalties, and significant damage to a company's reputation. For instance, a Boston hospital faced a $1 million settlement for violating HIPAA privacy rules due to lost documents, underscoring the financial repercussions of inadequate compliance measures.
The can be staggering. Organizations that fail to adhere to regulations may incur costs averaging $14.82 million, significantly exceeding the $5.47 million associated with compliant organizations. Furthermore, operational expenses linked to cleanup efforts can surpass compliance fines, further straining resources. In 2018, the SEC returned $794 million to investors affected by non-compliant companies, highlighting the financial risks tied to oversight failures.
Moreover, the erosion of public trust resulting from non-compliance can have long-lasting effects on a company's market position. A case study revealed that six hospitals and a nursing home were penalized $792,000 for failing to prevent unauthorized access to patient information, illustrating how regulatory failures can lead to both financial penalties and reputational harm. By upholding high standards of adherence, compliance professionals contribute to pharmaceutical regulatory affairs, supported by [AVS Life Sciences' expert consulting services](http://avslifesciences.com/blog-post/10-key-benefits-of-healthcare-compliance-consulting-for-pharma), which enhance the overall integrity of the drug industry, ensuring that patients receive safe and effective medications while safeguarding their organizations' interests.
Explore Current Trends and Future Directions in Regulatory Affairs
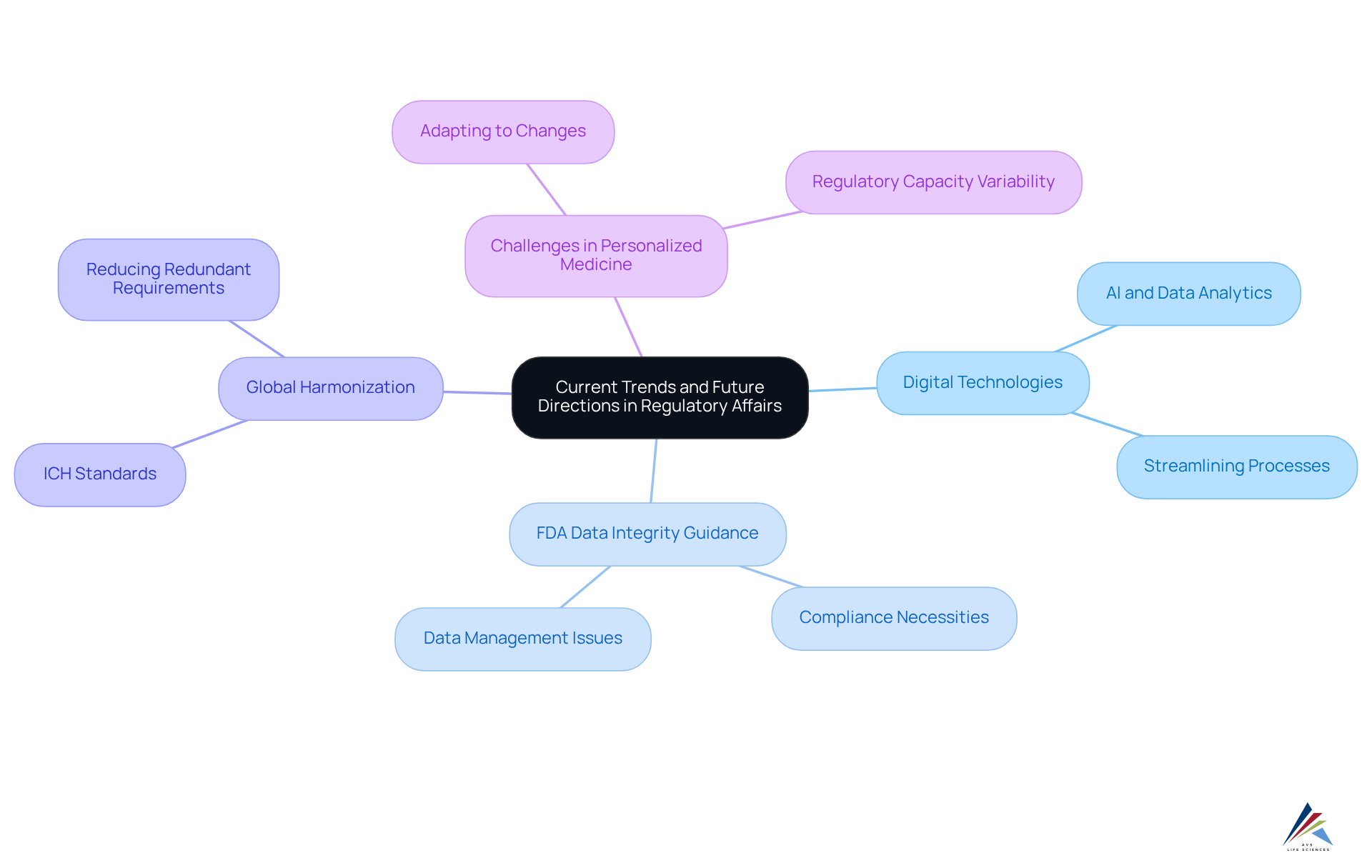
Current trends in compliance affairs underscore the transformative impact of digital technologies, particularly artificial intelligence and data analytics, which are streamlining processes and enhancing decision-making capabilities. The FDA's Data Integrity Guidance, effective since 2019, remains essential, as many companies continue to encounter citations for data management issues. This reality emphasizes the necessity of thoroughly understanding and effectively implementing these guidelines.
Furthermore, the global harmonization of pharmaceutical regulatory affairs is anticipated to simplify the approval process for products across various markets, promoting greater efficiency and consistency. For example, the International Conference on Harmonization (ICH) has played a pivotal role in aligning technical standards for drug efficacy, safety, and quality among major regions, significantly reducing redundant requirements.
The rise of personalized medicine and biologics introduces new challenges, compelling compliance experts to adapt to rapidly evolving scientific and oversight environments. AVS Life Sciences offers extensive adherence to regulations and validation solutions tailored to these challenges, ensuring that professionals are well-equipped to navigate the changing landscape.
As the industry evolves, it is imperative for professionals in pharmaceutical regulatory affairs to remain agile and proactive, safeguarding public health while ensuring compliance amidst these dynamic changes. To delve deeper into these topics, consider attending our upcoming webinar on the .
Conclusion
Understanding the intricacies of pharmaceutical regulatory affairs is essential for ensuring that medicinal products are safe, effective, and compliant with established regulations. This field plays a pivotal role in bridging the gap between drug innovation and regulatory compliance, safeguarding public health while fostering the integrity of the pharmaceutical industry.
Key insights from the discussion highlight the critical responsibilities of compliance specialists. These include:
- Monitoring legislation
- Managing submissions
- Ensuring adherence to safety standards throughout the product lifecycle
The historical evolution of regulatory affairs underscores the importance of stringent oversight, with past public health crises driving the establishment of robust compliance frameworks that continue to shape current practices.
As the pharmaceutical landscape evolves, embracing new technologies and global harmonization will be crucial for navigating the complexities of regulatory affairs. Professionals in this field must remain proactive and agile, adapting to emerging trends and challenges to uphold the highest standards of drug safety and efficacy. Engaging with resources and discussions surrounding these developments can empower organizations to enhance their compliance strategies, ultimately leading to better health outcomes for patients worldwide.
Frequently Asked Questions
What is the role of pharmaceutical regulatory affairs?
Pharmaceutical regulatory affairs is a specialized discipline focused on ensuring that medicinal products comply with established rules and guidelines. It involves preparing and submitting compliance documents, communicating with oversight authorities, and ensuring that products meet safety, efficacy, and quality standards.
Who are compliance specialists and what do they do?
Compliance specialists act as intermediaries between drug manufacturers and oversight organizations, ensuring adherence to legal and ethical standards throughout the medication development process. Their key duties include monitoring evolving legislation, providing guidance on legal and scientific obligations, and preparing registration papers for submission to oversight agencies.
Why is compliance important in the pharmaceutical industry?
Compliance is crucial for safeguarding public health and maintaining the integrity of the pharmaceutical industry. It ensures that products are safe and beneficial, and helps companies navigate complex regulations, thereby enhancing drug safety and efficacy.
How has the history of regulatory affairs evolved?
The history of drug oversight began with the Pure Food and Drug Act of 1906, aimed at eliminating misbranded or adulterated products. Subsequent legislation, like the Food, Drug, and Cosmetic Act of 1938, strengthened FDA authority and established rigorous standards for drug safety and approval. The evolution has been influenced by public health crises and technological advancements.
What historical events shaped pharmaceutical regulatory affairs?
Key events include the mass poisoning incident involving elixir sulfanilamide, which led to over 100 fatalities and prompted reforms for stricter regulations. Investigative journalism and studies by figures like Dr. Harvey Washington Wiley also played significant roles in raising public awareness and influencing regulatory practices.
What challenges do compliance professionals face today?
Compliance professionals navigate a complex landscape of international regulations, which is critical for achieving harmonization in the increasingly globalized pharmaceutical industry. They must adapt to evolving regulations while ensuring that quality management remains a priority in drug development.
How does AVS Life Sciences contribute to the field of regulatory affairs?
AVS Life Sciences is committed to providing comprehensive quality management and compliance solutions, enhancing the capabilities of compliance professionals. They leverage historical insights to inform current practices, ensuring that drug safety and effectiveness are prioritized.
