Understanding Pharmaceutical Compliance Consulting: Key Insights and Importance

Overview
Pharmaceutical compliance consulting is essential for pharmaceutical firms to navigate the complexities of regulatory standards and best practices, ensuring that their products consistently meet safety and efficacy requirements. These consulting services play a pivotal role in mitigating risks, enhancing operational efficiency, and fostering trust within the healthcare ecosystem. By addressing compliance challenges head-on, organizations can safeguard public health and safety while positioning themselves as leaders in their field. Engaging with AVS Life Sciences can empower firms to implement effective compliance solutions, ultimately contributing to a more robust healthcare landscape.
Introduction
Pharmaceutical compliance consulting is essential in safeguarding public health by ensuring that pharmaceutical companies adhere to stringent regulatory standards. By offering specialized guidance in critical areas such as Good Manufacturing Practices (GMP) and Quality System Regulations (QSR), these consulting services empower organizations to not only avoid costly penalties but also enhance their operational efficiency and bolster their reputation within the industry. As regulations evolve and the complexities of global supply chains increase, the pressing question arises: how can companies effectively navigate these challenges to maintain compliance and drive innovation?
Define Pharmaceutical Compliance Consulting and Its Importance
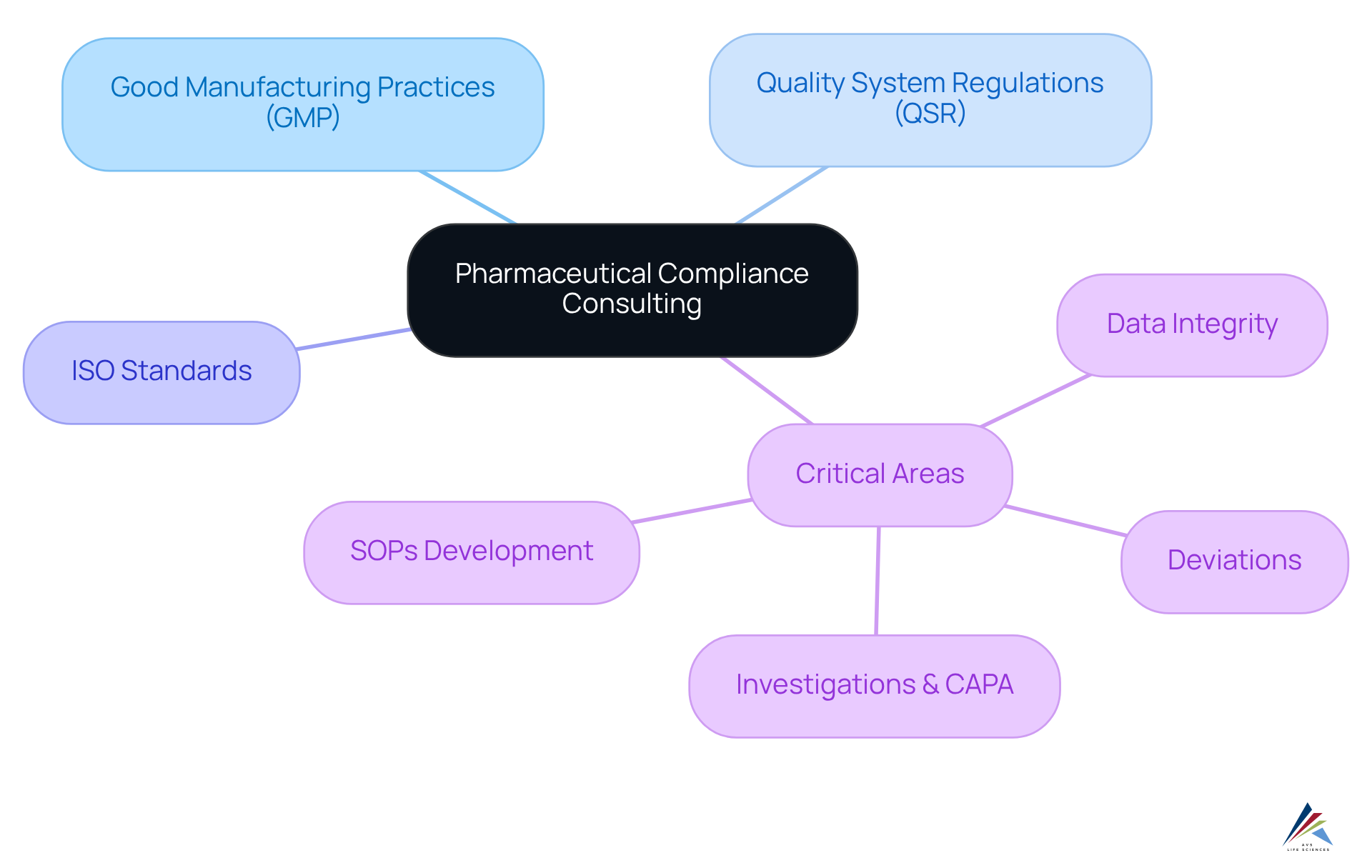
Pharmaceutical compliance consulting encompasses specialized advisory services designed to assist pharmaceutical firms in meeting regulatory standards and best practices. It provides detailed guidance on:
- Good Manufacturing Practices (GMP)
- Quality System Regulations (QSR)
- ISO standards
- Critical areas such as:
- Data Integrity
- Deviations
- Investigations & CAPA
- Standard Operating Procedures (SOPs) Development
with a particular focus on GXP adherence. AVS Life Sciences delivers expert solutions in GMP audits, ensuring compliance across APIs, drug products, and testing facilities.
Navigating the complexities of regulatory environments is vital for organizations, as pharmaceutical compliance consulting effectively mitigates risks and guarantees that products consistently meet safety and efficacy standards. Businesses that implement robust regulatory strategies not only avoid costly fines but also enhance their reputation within the sector. This, in turn, positively impacts public health and safety by ensuring that medications are manufactured according to the .
Surpassing basic regulatory requirements can significantly improve operational efficiency and foster trust among stakeholders, ultimately benefiting the healthcare ecosystem as a whole. By embracing pharmaceutical compliance consulting solutions, organizations can position themselves as leaders in the industry, driving both innovation and reliability in pharmaceutical practices.
Trace the Evolution of Pharmaceutical Compliance Consulting
The development of medication adherence consulting is intricately linked to the establishment of regulatory agencies and the implementation of stringent laws aimed at ensuring drug safety and effectiveness. Initially, adherence efforts primarily concentrated on manufacturing processes. However, as the pharmaceutical industry expanded and regulations grew more complex, the role of oversight advisors in pharmaceutical compliance consulting evolved significantly. They began to offer a wider array of services, including:
- Risk management
- Quality assurance
- Pharmaceutical compliance consulting
In contemporary practice, pharmaceutical compliance consulting has become a vital component of the drug lifecycle, adapting to innovations such as digital health and personalized medicine that necessitate the formulation of new regulatory frameworks. Historical case studies illustrate how oversight organizations have shaped the landscape of drug industry adherence, creating a demand for specialized advisory services capable of adeptly navigating intricate oversight environments. This ongoing evolution underscores the critical role of in upholding high standards of excellence and safety within the pharmaceutical sector.

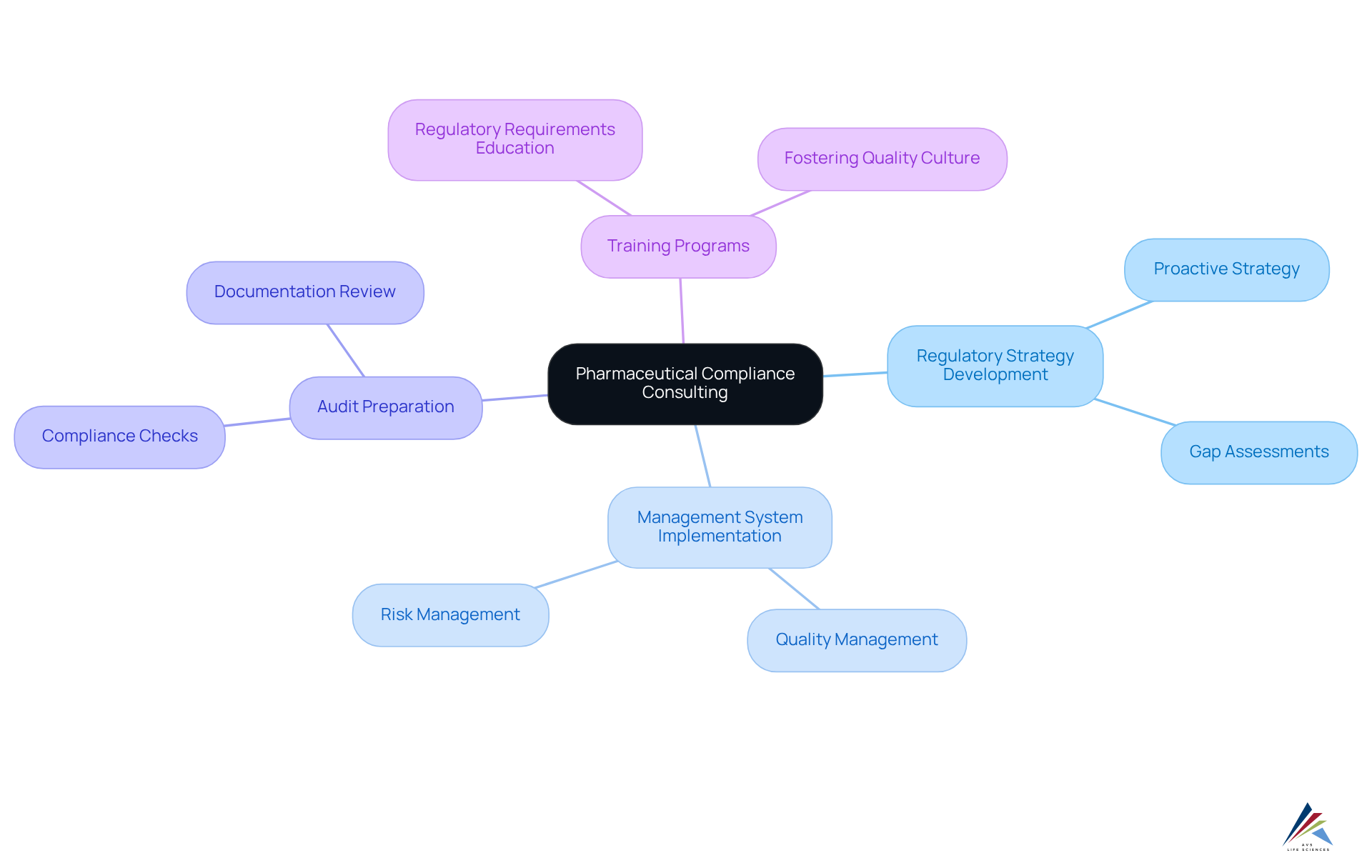
Identify Key Services and Components of Compliance Consulting
Essential services in pharmaceutical compliance consulting encompass:
- Regulatory strategy development
- Management system implementation
- Audit preparation
- Comprehensive training programs
Consultants are pivotal in pharmaceutical compliance consulting, aiding organizations to establish regulatory frameworks that not only comply with current regulations but also anticipate future changes. This proactive strategy involves conducting thorough to identify areas of non-compliance and providing actionable recommendations for enhancement.
Furthermore, training programs are crucial for equipping staff with a solid understanding of regulatory requirements, thereby fostering a culture of quality and accountability within organizations. As the pharmaceutical landscape evolves, integrating advanced technologies and innovative strategies into regulatory practices is increasingly essential, ensuring that companies remain competitive while navigating a complex regulatory environment.
Examine Challenges in Pharmaceutical Compliance Management
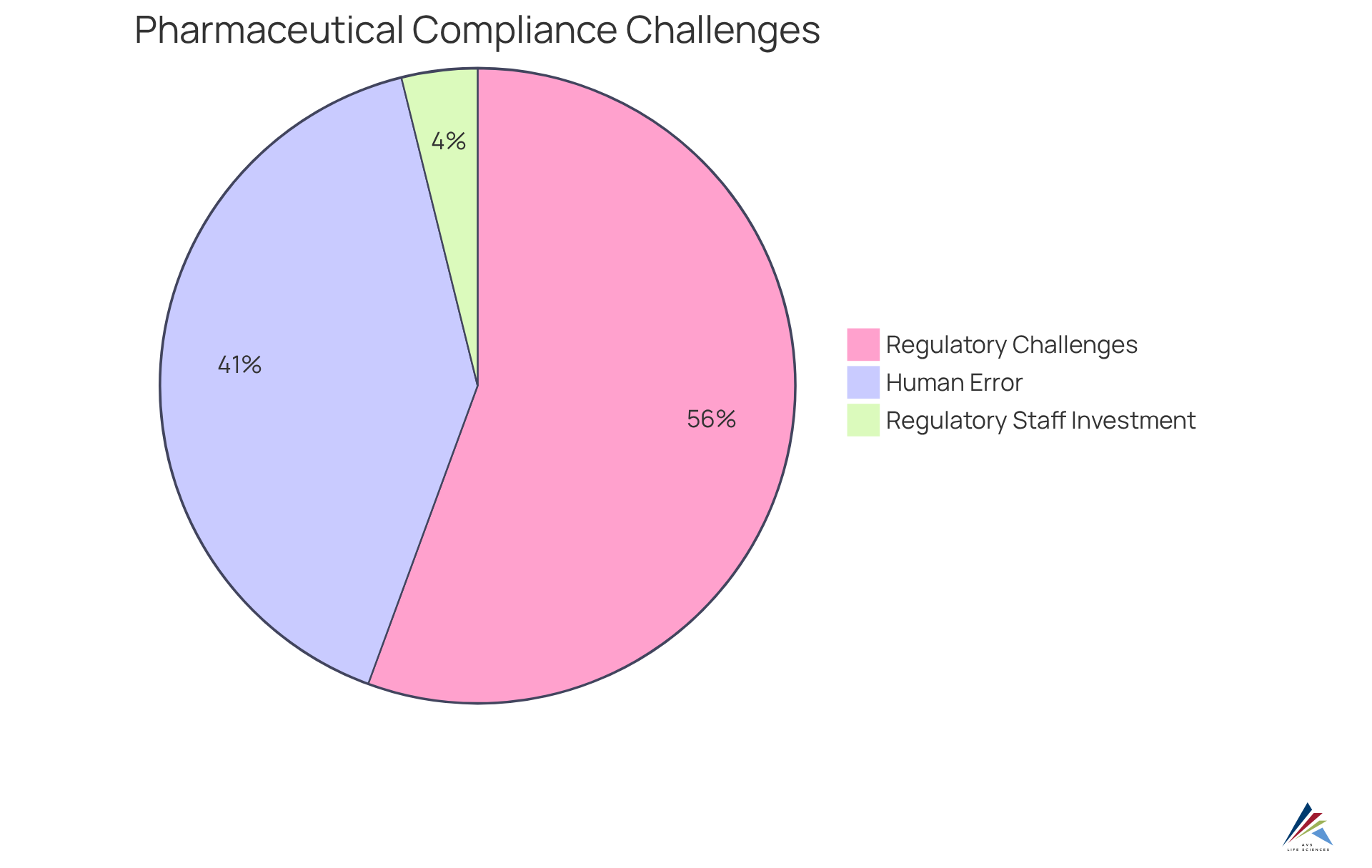
Pharmaceutical adherence management faces significant challenges due to rapidly evolving regulations, intricate global supply chains, and rising adherence costs. A striking 72% of pharmaceutical quality professionals report difficulties in adapting to these changing requirements, which can inadvertently lead to regulatory violations. The complexities of global supply chains further complicate adherence efforts, as varying regional regulations necessitate customized strategies. Additionally, human error, often stemming from insufficient training, accounts for 40% to 65% of major Good Manufacturing Practice (GMP) deviations. To effectively navigate these challenges, organizations must prioritize investments in:
- Pharmaceutical compliance consulting
- Comprehensive regulatory programs
- Continuous training initiatives
- Proactive risk management strategies
For example, AVS Life Sciences successfully assisted a leading biotechnology company in upgrading their manufacturing facility from a Biosafety Level 1 GMP to a Level 2 GMP. This partnership not only ensured but also allowed the client to focus on developing medicines that enhance patient well-being. The project was completed on time and within budget, demonstrating the effectiveness of AVS's management solutions. Such instances underscore the importance of viewing adherence not merely as a regulatory obligation but as a strategic advantage that can be achieved through pharmaceutical compliance consulting, ultimately enhancing operational efficiency and product quality.
Moreover, businesses typically invest $3-5 million annually on regulatory staff, highlighting the financial implications of maintaining compliance standards. As Scott Gottlieb aptly noted, "The companies that succeed won’t be those that do the minimum necessary for adherence, but those that embrace quality as their competitive advantage." Additionally, it is noteworthy that 39% of FDA observations can be classified into just ten areas, illustrating common compliance challenges within the industry. Addressing these issues through proactive risk management strategies is essential for achieving long-term success in pharmaceutical compliance consulting.
Conclusion
Pharmaceutical compliance consulting stands as a critical pillar in ensuring that pharmaceutical companies adhere to complex regulatory standards and best practices. By providing specialized guidance on key areas such as Good Manufacturing Practices (GMP) and Quality System Regulations (QSR), these consulting services not only help organizations mitigate risks but also enhance their operational efficiency and reputation within the industry. The emphasis on compliance ultimately contributes to public health by ensuring that medications are produced to the highest quality standards.
Throughout this article, various aspects of pharmaceutical compliance consulting have been explored, including its evolution, essential services, and the challenges organizations face in maintaining compliance. The discussion highlighted how the role of compliance consultants has expanded from merely advising on manufacturing processes to offering comprehensive regulatory strategies that anticipate future changes. Furthermore, the importance of continuous training and proactive risk management was underscored as vital components for navigating the complexities of compliance in a rapidly changing regulatory landscape.
In light of these insights, it is evident that embracing pharmaceutical compliance consulting transcends mere regulatory obligations; it represents a strategic advantage that empowers organizations to thrive in a competitive market. As the pharmaceutical industry continues to evolve, prioritizing compliance will be paramount for ensuring product quality, operational excellence, and ultimately, the well-being of patients. Organizations are encouraged to invest in robust compliance strategies and expert consulting services to navigate the challenges ahead and emerge as leaders in the pharmaceutical sector.
Frequently Asked Questions
What is pharmaceutical compliance consulting?
Pharmaceutical compliance consulting refers to specialized advisory services that help pharmaceutical firms meet regulatory standards and best practices, focusing on areas such as Good Manufacturing Practices (GMP), Quality System Regulations (QSR), ISO standards, and critical issues like data integrity, deviations, investigations, and the development of Standard Operating Procedures (SOPs.)
Why is pharmaceutical compliance consulting important?
It is important because it helps organizations navigate complex regulatory environments, mitigates risks, and ensures that products meet safety and efficacy standards. This not only avoids costly fines but also enhances the company's reputation and positively impacts public health and safety.
What specific areas does pharmaceutical compliance consulting cover?
It covers areas including Good Manufacturing Practices (GMP), Quality System Regulations (QSR), ISO standards, data integrity, deviations, investigations and CAPA (Corrective and Preventive Actions), and the development of Standard Operating Procedures (SOPs).
How does AVS Life Sciences contribute to pharmaceutical compliance?
AVS Life Sciences provides expert solutions in GMP audits, ensuring compliance across Active Pharmaceutical Ingredients (APIs), drug products, and testing facilities.
What are the benefits of implementing robust regulatory strategies in pharmaceutical firms?
Implementing robust regulatory strategies can improve operational efficiency, foster trust among stakeholders, enhance the firm’s reputation, and ultimately benefit the healthcare ecosystem by ensuring high-quality medication manufacturing.
How can organizations position themselves as leaders in the pharmaceutical industry?
By embracing pharmaceutical compliance consulting solutions, organizations can drive innovation and reliability in their practices, thus positioning themselves as leaders in the industry.
