Understanding CRO Meaning in Pharma for Compliance Officers

Overview
The article emphasizes the pivotal role and significance of Contract Research Organizations (CROs) within the pharmaceutical industry, particularly for compliance officers. It asserts that CROs deliver essential outsourced research services that streamline clinical study processes, enhance regulatory compliance, and ultimately accelerate drug development. Supported by data illustrating their growing market demand and expertise in navigating complex regulatory landscapes, the article underscores the necessity for compliance solutions in an evolving industry.
Introduction
Understanding the role of Contract Research Organizations (CROs) in the pharmaceutical industry has become increasingly vital as the landscape of drug development evolves. These specialized entities not only streamline clinical studies but also ensure compliance with rigorous regulatory standards, allowing pharmaceutical companies to concentrate on their core missions.
However, the rise of decentralized clinical trials and the complexities of global regulations present significant compliance challenges. How can compliance officers effectively navigate these hurdles while maximizing the benefits of CRO partnerships?
This article delves into the multifaceted role of CROs, exploring their significance in enhancing compliance and driving innovation in pharmaceutical research.
Define Contract Research Organization (CRO) in Pharma
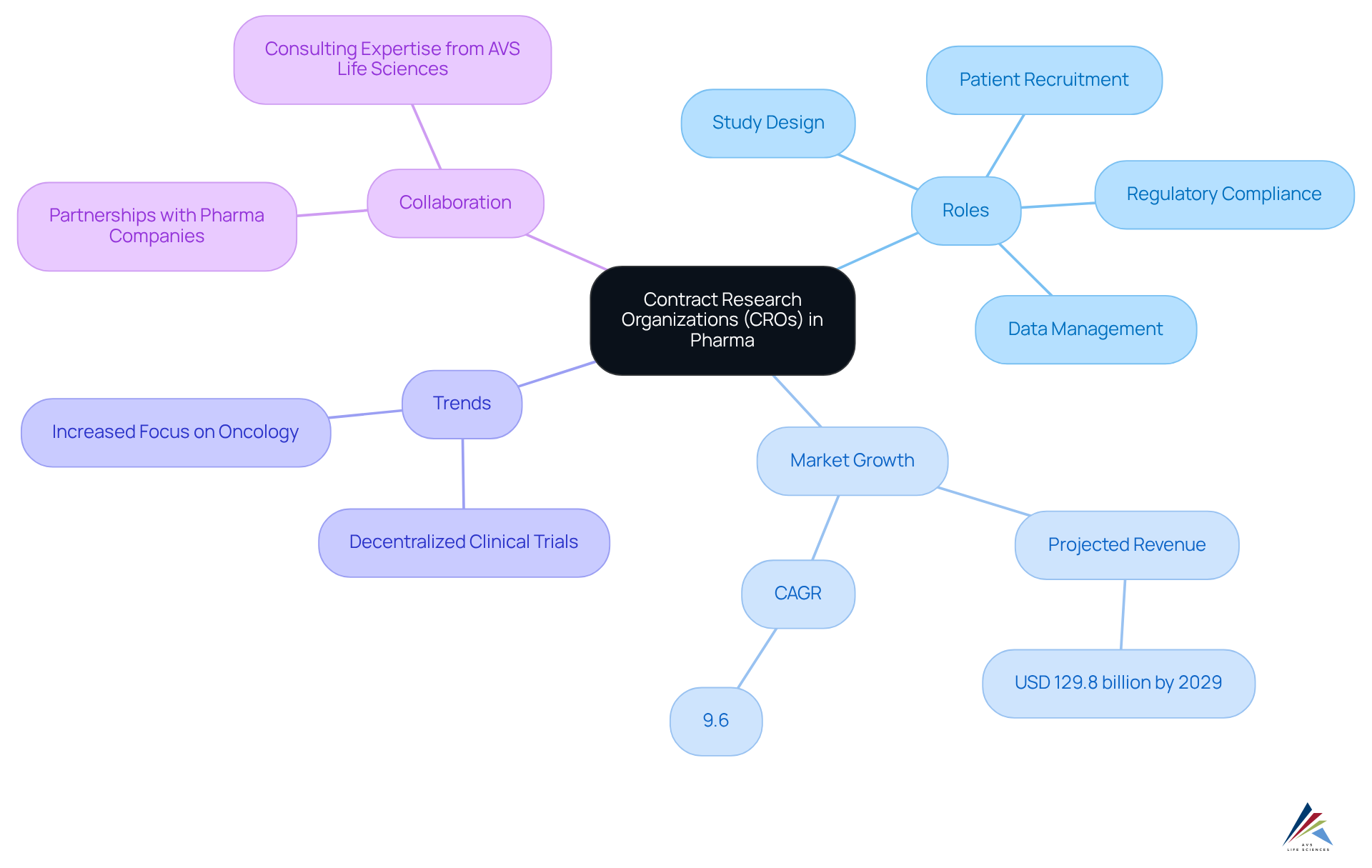
A Contract Research Organization (CRO) meaning pharma serves as a specialized entity that provides outsourced research services to the pharmaceutical, biotechnology, and medical device sectors. By 2025, the number of CROs meaning pharma operating within the pharmaceutical sector is expected to grow significantly, reflecting an increasing reliance on these organizations for effective clinical study management. CROs oversee critical aspects of clinical studies, including:
- Study design
- Patient recruitment
- Data management
- Regulatory compliance
This enables pharmaceutical companies to concentrate on their core competencies while ensuring that studies are conducted efficiently and adhere to regulatory standards.
Recent trends reveal a shift towards decentralized clinical studies, which improve patient recruitment and retention by offering enhanced flexibility. The CRO services market is projected to reach USD 129.8 billion by 2029, with a compound annual growth rate (CAGR) of 9.6%, highlighting the escalating demand for these services. Notably, the oncology sector exemplifies effective CRO involvement in clinical studies, having led the market in 2023 due to increased funding and a rising incidence of cancer.
Experts assert that CRO meaning pharma are pivotal in pharmaceutical studies, facilitating faster drug development and improving compliance with guidelines. Their specialized expertise and resources, combined with AVS Life Sciences' consulting proficiency and pragmatic approach, are vital for navigating the complexities of clinical studies. This collaboration ultimately leads to more effective and timely therapeutic solutions. AVS Life Sciences offers comprehensive quality management and compliance solutions, essential for CROs striving to meet high-quality benchmarks and compliance requirements in a competitive landscape.
Explore the Role of CROs in Pharmaceutical Development
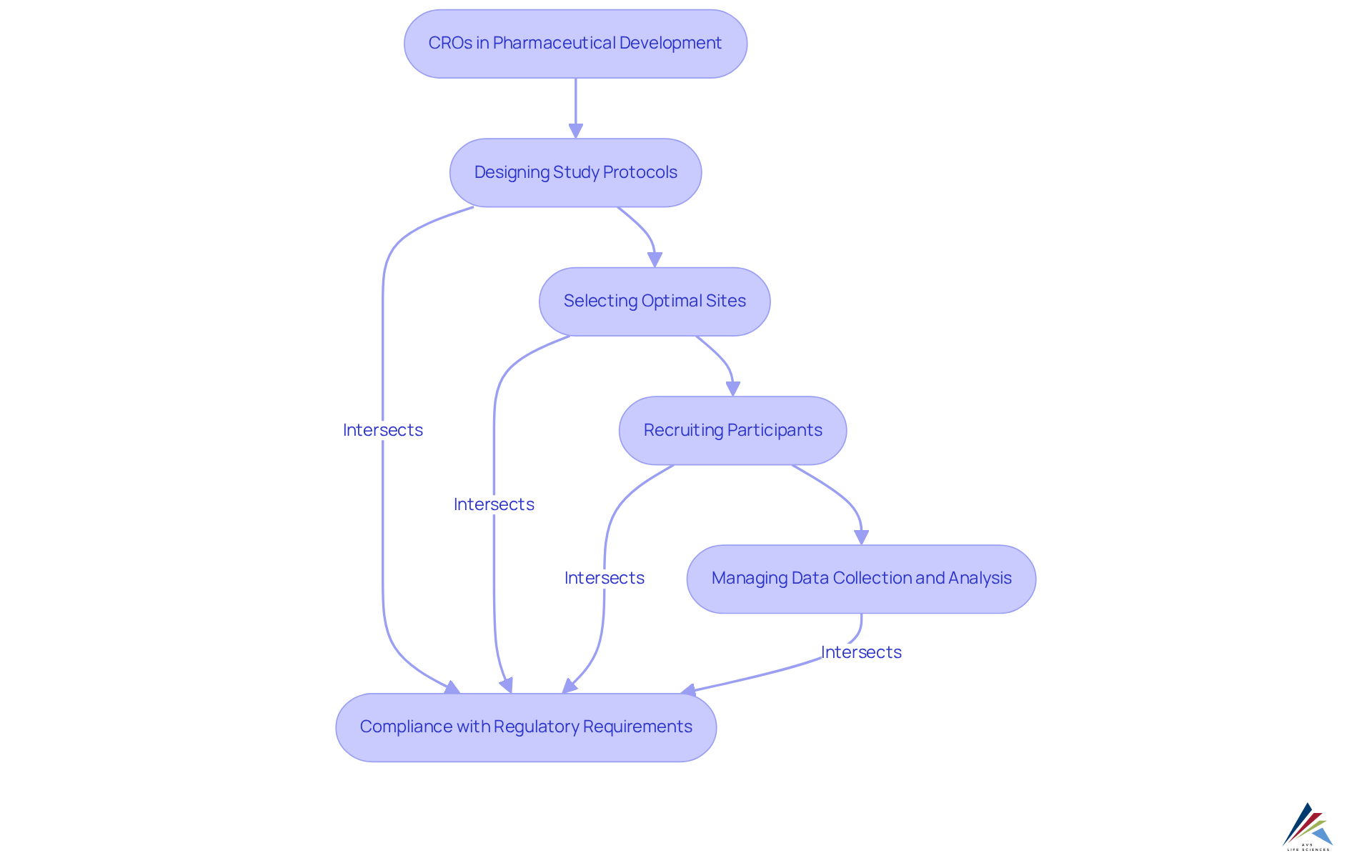
CROs, or cro meaning pharma, are essential to pharmaceutical advancement, offering a comprehensive range of services that significantly streamline the clinical study process. They play a pivotal role in:
- Designing study protocols
- Selecting optimal sites
- Recruiting participants
- Managing data collection and analysis
By ensuring compliance with regulatory requirements, including GXP and FDA regulations, CROs prepare and submit necessary documentation to regulatory bodies such as the FDA and EMA, adeptly navigating complex regulatory landscapes. This expertise not only mitigates risks but also enhances the likelihood of successful drug approvals.
The time saved in clinical studies by employing CROs can be substantial, with reports indicating that they can accelerate site activation and patient recruitment, leading to quicker study completion. For instance, the integration of cutting-edge technologies and data analysis by CROs has been shown to improve operational efficiencies, enabling rapid modifications to study designs based on real-time information and addressing potential data integrity issues.
Pharmaceutical executives increasingly recognize the strategic value of CROs, understanding the concept of cro meaning pharma, and view them as partners rather than mere service providers. One executive noted, "Collaborating with a CRO has enabled our company to concentrate on core strengths while utilizing the CRO's expertise in clinical research management." This collaboration fosters enhanced communication and alignment of objectives, ultimately improving the effectiveness of clinical trials.
A transformative example of this partnership is illustrated by AVS Life Sciences' successful upgrade of a biotechnology GMP facility. By assisting a prominent San Francisco-based biotechnology firm in transitioning from a Biosafety Level 1 GMP facility to a Level 2 GMP facility, AVS Life Sciences demonstrated the critical importance of quality assurance and regulatory compliance in the CRO landscape. Their meticulous documentation practices and adherence to standard operating procedures (SOPs) ensured full traceability, validated by the client’s quality assurance team. This collaboration enabled the client to focus on developing medicines while AVS Life Sciences managed the complexities of compliance and quality control.
As the landscape of clinical studies evolves, CROs, which are pivotal in the CRO meaning pharma, continually adapt by incorporating innovative methods and technologies to meet the growing demands of the pharmaceutical sector. Their role as intermediaries between sponsors and regulatory authorities not only facilitates communication but also ensures that studies adhere to ethical standards, further solidifying their significance in the drug development process.
Examine Regulatory Compliance and Challenges for CROs
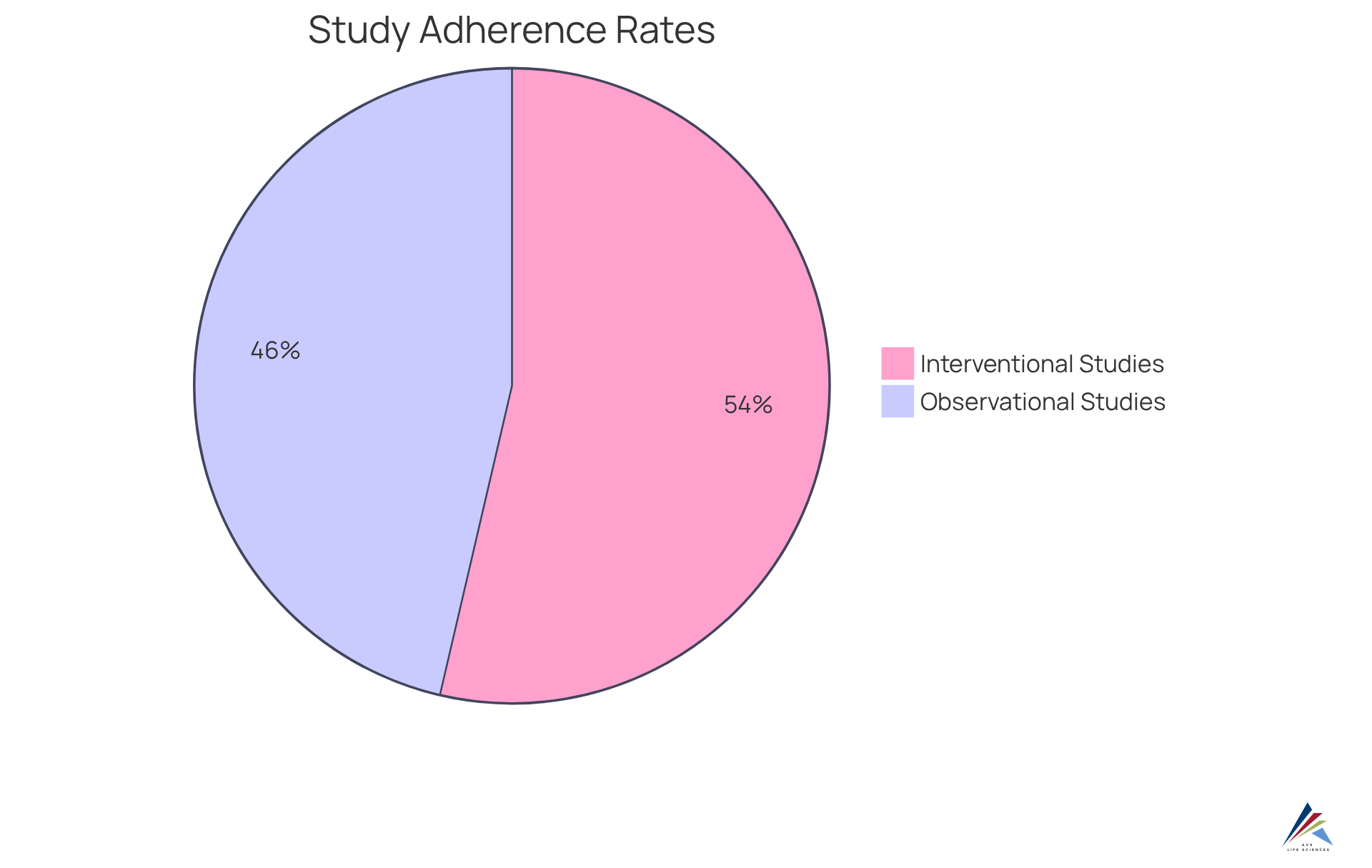
Clinical research organizations operate within a rigorously regulated environment, facing significant adherence challenges that demand strict compliance with Good Clinical Practice (GCP) guidelines as well as local and international regulations governing clinical trials. Notably, adherence rates for interventional studies among CROs exceed 92%, while observational studies lag at approximately 79.5%. This discrepancy underscores the critical importance of robust regulatory mechanisms. Common challenges include:
- Managing protocol deviations
- Ensuring data integrity
- Upholding ethical standards throughout the research process
Additionally, clinical research organizations must navigate diverse compliance requirements across various countries, complicating the execution and adherence of trials.
To effectively tackle these challenges, many CROs adopt comprehensive quality management systems designed to enhance adherence to GCP guidelines. Routine evaluations and practice assessments are integral to these systems, aiding in the identification of weaknesses and ensuring that all processes meet compliance expectations. For instance, organizations like bioaccess have successfully accelerated approvals and improved patient enrollment through effective adherence strategies. The capacity to manage these complexities is essential for preserving the integrity of clinical trials and protecting participant rights, ultimately fostering trust within the research community. As one regulatory officer aptly noted, "Maintaining transparency and honesty in our operations is vital for guaranteeing adherence to ethical and regulatory principles." This commitment to quality and adherence not only protects participants but also enhances the credibility of the research conducted by Contract Research Organizations.
Identify Benefits of Outsourcing to CROs for Compliance
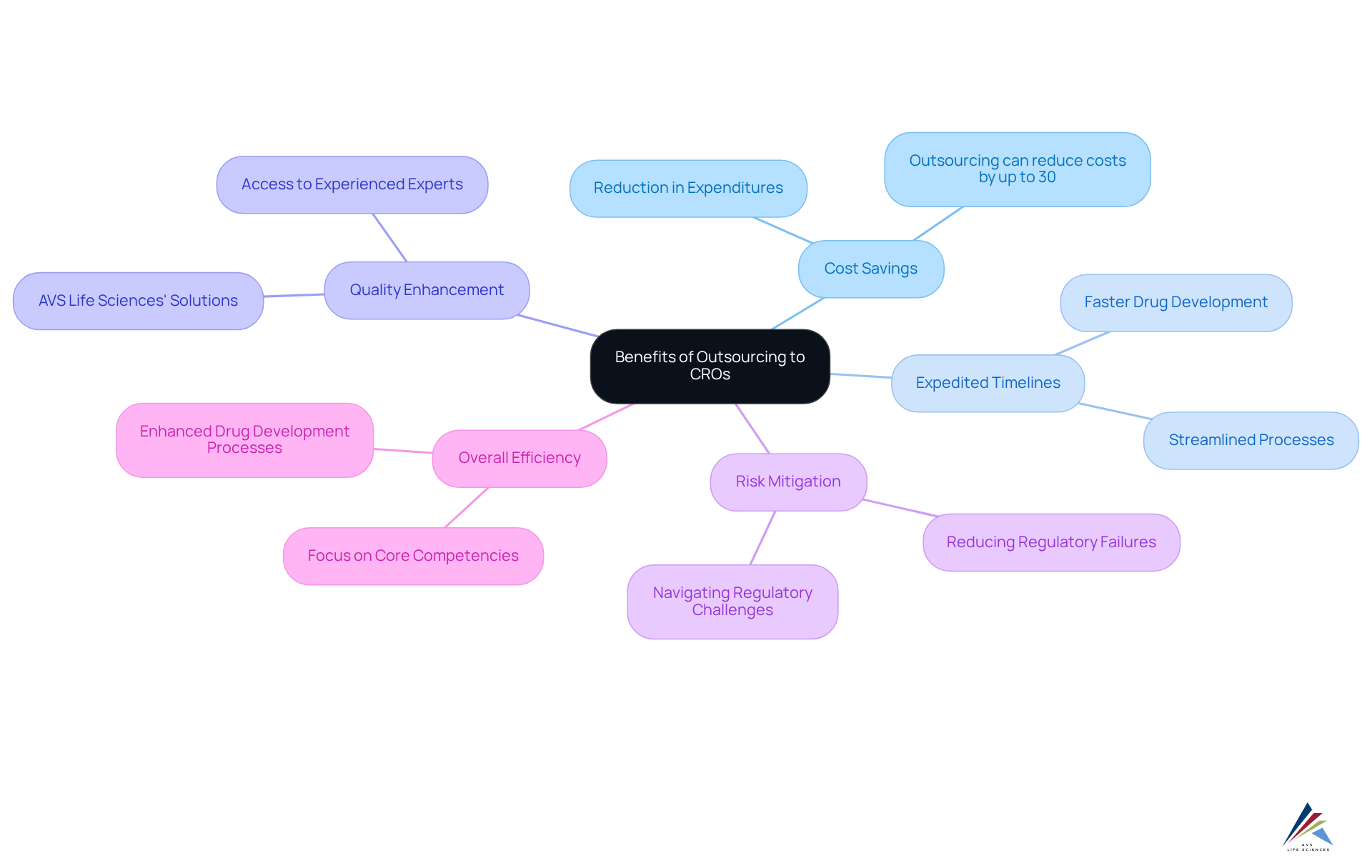
Utilizing Contract Research Organizations (CROs), or cro meaning pharma, presents significant advantages for pharmaceutical companies, especially in the realm of regulatory management. By leveraging the specialized knowledge of CROs meaning pharma, companies can achieve substantial operational cost savings, expedite timelines, and elevate the quality of their clinical studies. Access to seasoned experts with a deep understanding of regulatory requirements enables sponsors to navigate the intricate regulatory landscape more effectively, reflecting the cro meaning pharma.
This strategic collaboration empowers pharmaceutical firms to focus on their core competencies while ensuring that clinical trials meet the highest standards of quality and ethical practices. Moreover, engaging with CRO partnerships, reflecting the cro meaning pharma, not only mitigates risks associated with regulatory failures but also enhances the overall efficiency of the drug development process.
For instance, AVS Life Sciences offers comprehensive quality management and regulatory adherence solutions that can significantly bolster pharmaceutical firms' CRO partnerships. Companies that have engaged contract research organizations report notable cost savings, with some estimates suggesting that outsourcing can reduce expenditures by as much as 30%.
As the global healthcare CRO market is anticipated to expand at a compound annual growth rate of 7.02% from 2025 to 2030, the trend of employing CROs for compliance and operational support, which is often associated with cro meaning pharma, is poised to gain further momentum.
Conclusion
CROs, or Contract Research Organizations, are essential to the pharmaceutical industry, providing specialized research services that significantly enhance the efficiency and compliance of clinical studies. Their growing importance is highlighted by the increasing reliance on their expertise, enabling pharmaceutical companies to concentrate on core competencies while ensuring that clinical trials adhere to regulatory standards.
Key insights throughout the article reveal the multifaceted functions of CROs, including:
- Study design
- Patient recruitment
- Data management
This discussion underscores the critical nature of regulatory compliance, illustrating how CROs adeptly navigate complex regulatory landscapes and mitigate risks associated with clinical trials. The anticipated growth of the CRO market further emphasizes their vital role in accelerating drug development and improving overall operational efficiencies.
Engaging with CROs is not merely a strategic advantage; it has become a necessity for pharmaceutical companies aiming to thrive in a competitive environment. As the clinical research landscape continues to evolve, embracing partnerships with CROs will be crucial for ensuring compliance, enhancing the quality of drug development, and ultimately delivering effective therapeutic solutions to patients. The future of pharmaceutical research is undoubtedly intertwined with the expertise and capabilities that CROs bring to the table.
Frequently Asked Questions
What is a Contract Research Organization (CRO) in the pharmaceutical industry?
A Contract Research Organization (CRO) in pharma is a specialized entity that provides outsourced research services to the pharmaceutical, biotechnology, and medical device sectors, focusing on effective clinical study management.
What services do CROs provide in clinical studies?
CROs oversee critical aspects of clinical studies, including study design, patient recruitment, data management, and regulatory compliance.
How do CROs benefit pharmaceutical companies?
CROs enable pharmaceutical companies to focus on their core competencies while ensuring that studies are conducted efficiently and adhere to regulatory standards.
What is the projected growth of the CRO services market?
The CRO services market is projected to reach USD 129.8 billion by 2029, with a compound annual growth rate (CAGR) of 9.6%.
What trends are emerging in clinical studies?
There is a shift towards decentralized clinical studies, which improve patient recruitment and retention by offering enhanced flexibility.
Which sector exemplifies effective CRO involvement in clinical studies?
The oncology sector exemplifies effective CRO involvement in clinical studies, having led the market in 2023 due to increased funding and a rising incidence of cancer.
Why are CROs considered pivotal in pharmaceutical studies?
CROs are pivotal in pharmaceutical studies because they facilitate faster drug development and improve compliance with guidelines through their specialized expertise and resources.
What role does AVS Life Sciences play in relation to CROs?
AVS Life Sciences offers comprehensive quality management and compliance solutions, which are essential for CROs striving to meet high-quality benchmarks and compliance requirements in a competitive landscape.
