Understanding CMC Pharma Meaning: Key Concepts and Importance

Overview
The term "CMC" in pharmaceuticals denotes Chemistry, Manufacturing, and Controls—critical components that ensure the quality, safety, and efficacy of medications during their development and production. A comprehensive understanding of CMC practices is imperative for regulatory compliance, as the landscape of pharmaceutical manufacturing becomes increasingly complex. The significance of robust CMC submissions cannot be overstated; they are essential for successful drug approval. As regulations evolve, the industry must adapt, reinforcing the necessity for high standards in CMC practices. Engaging with these practices not only meets compliance challenges but also positions organizations for success in a competitive environment.
Introduction
Understanding the intricacies of Chemistry, Manufacturing, and Controls (CMC) is essential for anyone navigating the pharmaceutical landscape. This critical framework governs not only the development and production of medications but also plays a pivotal role in ensuring patient safety and product efficacy. As the industry confronts evolving regulatory demands and the increasing complexity of biologics, the significance of CMC becomes even more pronounced.
How can pharmaceutical companies effectively adapt their CMC strategies to meet these challenges while maintaining compliance and quality? This question underscores the urgent need for innovative solutions in compliance management.
Define CMC in Pharmaceuticals: Core Concepts and Importance
The term cmc pharma meaning refers to Chemistry, Manufacturing, and Controls (CMC), which is an essential component of pharmaceutical applications, delineating the processes integral to medication development and production. This section encompasses the substance's chemical composition, manufacturing processes, control measures for standards, and stability assessments. The CMC pharma meaning goes beyond mere regulatory approval; it is critical for ensuring that pharmaceutical products are consistently produced and controlled in accordance with stringent quality standards. Understanding the cmc pharma meaning is vital for maintaining compliance throughout the product lifecycle, directly impacting patient safety and medication efficacy.
Recent advancements in CMC regulations highlight its dynamic nature. For example, the FDA's Chemistry, Manufacturing, and Controls Development and Readiness Pilot (CDRP) Program, launched in 2022, seeks to accelerate CMC development for products under investigational new drug (IND) applications. This initiative promotes enhanced communication between the FDA and sponsors, facilitating earlier patient access to innovative therapies. With the biologics market anticipated to expand at a compound annual growth rate of 15% until 2027, the cmc pharma meaning becomes increasingly critical, especially for complex biologics such as monoclonal antibodies.
AVS Life Sciences has cultivated a reputation for delivering rapid value creation and meticulous project execution, achieving an impressive 80% repeat business rate. Their expertise in CMC, particularly through comprehensive GMP audits, highlights the cmc pharma meaning and ensures that clients adeptly navigate the intricacies of compliance. A recent project exemplifies this, showcasing how AVS Life Sciences assisted a client in optimizing their CMC processes, culminating in on-time project delivery and zero findings during audits.
In conclusion, the cmc pharma meaning encompasses a comprehensive strategy that is not merely essential for securing approval; it is also pivotal for safeguarding patient safety and ensuring the efficacy of medications. As the pharmaceutical landscape continues to evolve, the CMC pharma meaning will remain central to the successful development and commercialization of new therapies.
Trace the Evolution of CMC: Historical Context and Development
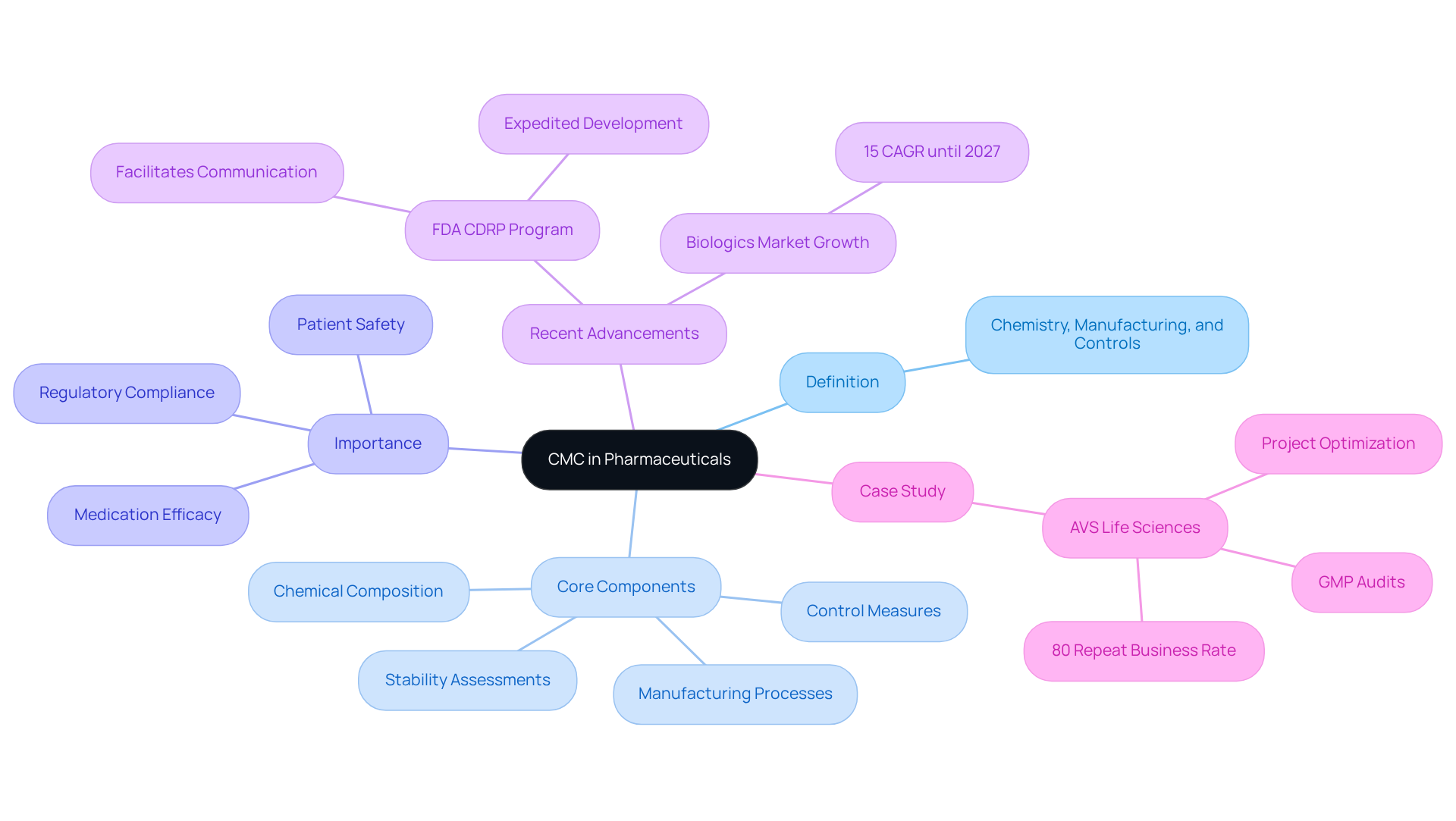
The evolution of the concept known as CMC pharma meaning has been substantial over the years, driven by heightened oversight and the demand for enhanced quality standards in pharmaceutical production. Initially, CMC represented a straightforward aspect of drug development, focusing primarily on basic chemical composition and manufacturing processes. However, as the pharmaceutical landscape has broadened, regulatory authorities such as the FDA and EMA have instituted increasingly rigorous guidelines, significantly complicating the CMC pharma meaning and its requirements.
The implementation of Good Manufacturing Practices (GMP) and Quality by Design (QbD) principles has been crucial in redefining the CMC framework. GMP encompasses the necessary methods to ensure that products are consistently manufactured and regulated in accordance with established standards, while QbD emphasizes the integration of excellence into the product from the outset, rather than relying solely on final product evaluations. These standards underscore the importance of a holistic approach to excellence and risk management throughout the drug development lifecycle.
A transformative case study involving AVS Life Sciences effectively illustrates this evolution. The company supported a leading biotechnology firm in upgrading its manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This upgrade entailed the implementation of advanced assurance processes and meticulous documentation practices to guarantee compliance with standards. The project was completed on schedule and within budget, highlighting the critical role of robust strategies that reflect the CMC pharma meaning in ensuring that products meet standards while maintaining excellence from clinical trials to market.
Throughout the upgrade, the team encountered challenges, such as ensuring the proper installation of equipment and addressing discrepancies in test results due to overlooked barcode scanner configurations. These challenges underscored the necessity for comprehensive testing and validation processes. Statistics reveal that approximately 94 molecules experience attrition from early-stage development, indicating that only about 6 out of 100 molecules transition from the discovery stage to phase III clinical trials. This statistic emphasizes the importance of thorough CMC strategies, which relate to CMC pharma meaning, such as those provided by AVS Life Sciences, in navigating the complexities of compliance and securing timely approvals. As regulatory scrutiny intensifies, the pharmaceutical industry must adapt its CMC practices to align with the CMC pharma meaning, ensuring compliance and enhancing product quality.
Identify Key Components of CMC: Characteristics and Functions
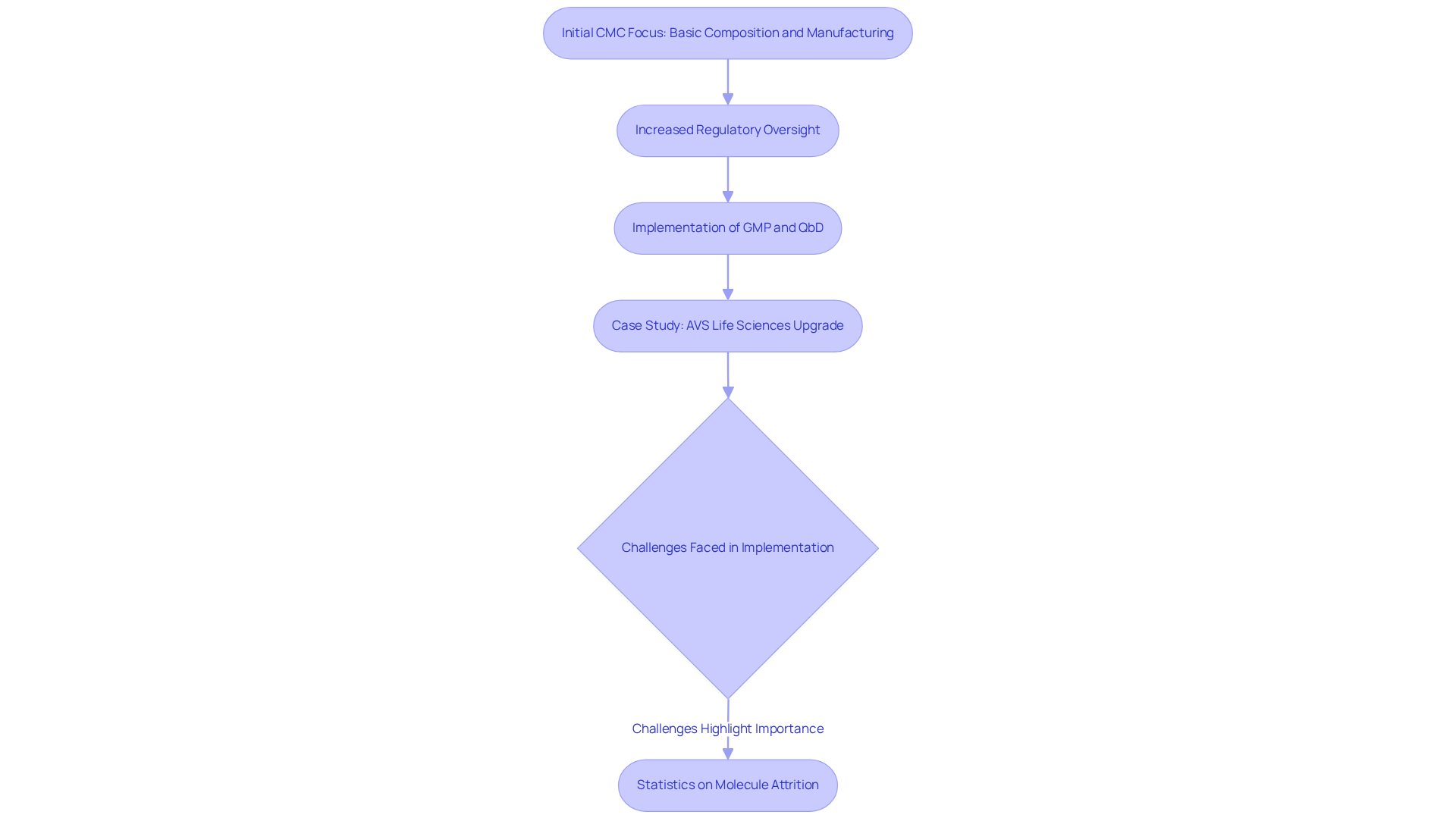
Key components of CMC include the following:
-
Chemistry: A comprehensive overview of the substance is essential, outlining its chemical structure, properties, and formulation. The significance of chemistry in medication formulation is underscored by statistics indicating that effective chemistry practices can enhance success rates in pharmaceutical development.
-
Manufacturing: This section delineates the processes employed to create the medicine, encompassing the equipment, facilities, and production methods utilized. Current manufacturing process standards in the pharmaceutical industry stress the necessity of compliance with Good Manufacturing Practices (GMP) to ensure product quality and safety. AVS Life Sciences plays a pivotal role in guiding pharmaceutical manufacturers through the complexities of GMP compliance, as evidenced by their successful transition of a facility from Biosafety Level 1 to Level 2 GMP standards. The FDA's 2011 Process Validation Guidance further emphasizes the critical nature of continued process verification (CPV) as a mandatory requirement for regulated industries.
-
Controls: Quality control measures are paramount to guarantee that the drug adheres to specified standards throughout its lifecycle. This includes rigorous testing protocols, stability studies, and batch release criteria, all essential for maintaining product integrity. Moreover, data integrity and Corrective and Preventive Actions (CAPA) are vital elements that ensure adherence to compliance expectations. AVS Life Sciences guarantees the effective execution of these controls, assisting clients in maintaining compliance.
-
Documentation: Comprehensive documentation is indispensable for demonstrating adherence to legal requirements and facilitating audits. This encompasses standard operating procedures (SOPs), batch records, and validation reports. Efficient documentation methods are crucial for ensuring that all CMC activities are traceable and fulfill compliance expectations, particularly in light of the cmc pharma meaning regarding the necessity for thorough assessments of excipient changes. AVS Life Sciences emphasizes the importance of robust documentation practices, enabling clients to navigate audits and maintain compliance seamlessly.
Each of these elements plays a crucial role in ensuring that pharmaceutical products are safe, effective, and of high standard, ultimately contributing to the overall success of medication development initiatives.
Explain the Role of CMC in Regulatory Compliance: Ensuring Quality and Safety
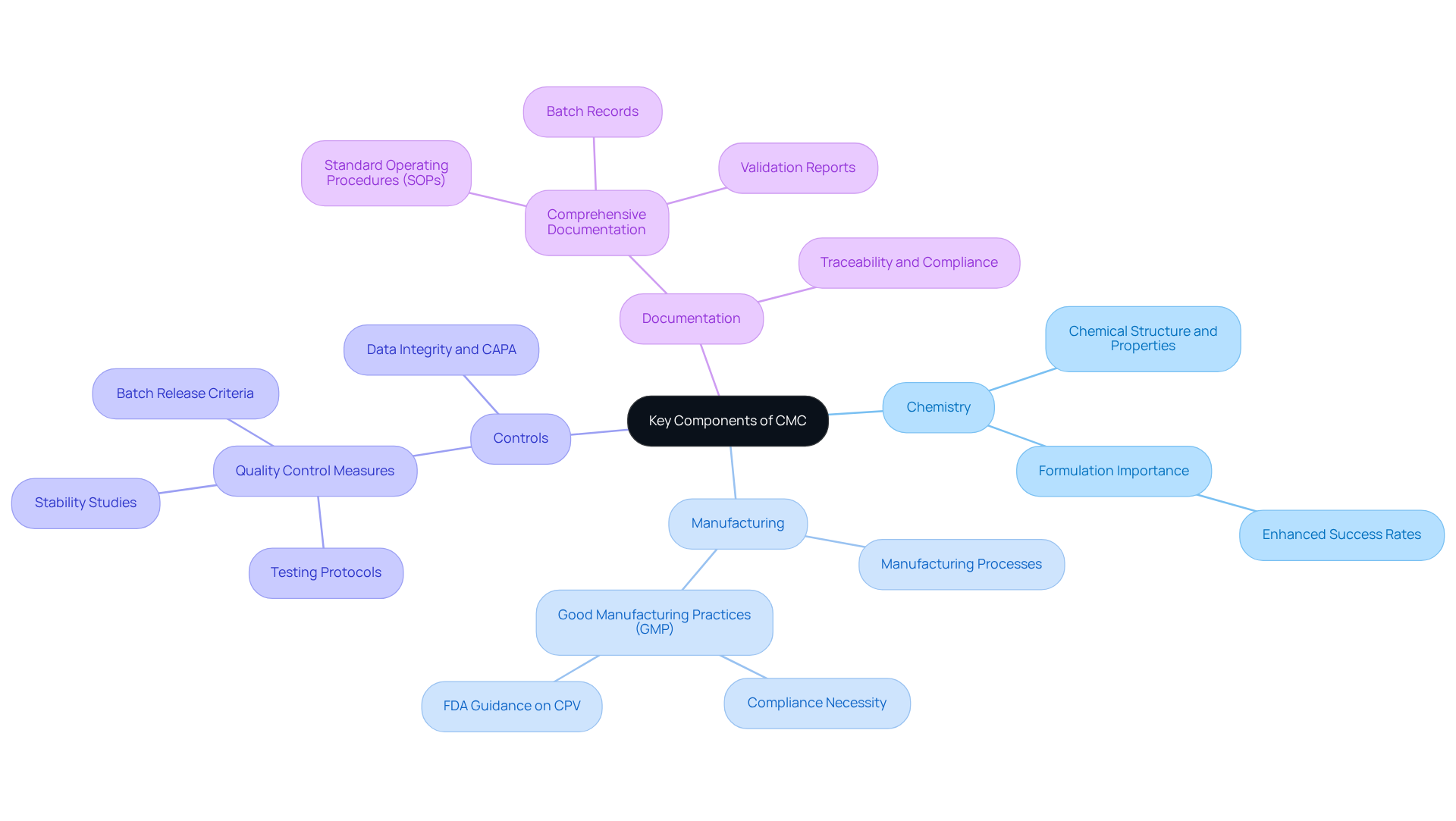
CMC is essential for adherence to guidelines, ensuring that every facet of medicine development and production aligns with rigorous standards. Regulatory bodies mandate a comprehensive CMC submission as part of the drug approval process, which is critical for demonstrating that products can be consistently produced to meet defined standards. This submission must encompass thorough evidence of stability, purity, and potency, all of which are vital for regulatory approval.
Furthermore, CMC documentation plays a significant role during inspections and audits, providing a transparent overview of the manufacturing processes and quality controls implemented. By strictly adhering to CMC guidelines, pharmaceutical companies can effectively mitigate risks associated with non-compliance, including product recalls, legal repercussions, and reputational damage. Notably, the FDA has issued over 15,749 recalls of medications since 2012, highlighting the importance of robust CMC practices in preventing such occurrences.
Companies with well-structured CMC submissions not only experience higher approval rates but also enjoy shorter timelines, reinforcing the necessity of these submissions for successful drug approval. A transformative case study involving AVS Life Sciences illustrates this point: the company successfully assisted a leading biotechnology firm in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This upgrade ensured compliance with higher quality standards and enhanced the client's operational efficiency and quality assurance processes, allowing them to concentrate on developing medicines that improve patient quality of life.
Expert insights indicate that understanding the nuances of CMC pharma meaning can significantly influence product launch success, making it a critical focus for pharmaceutical compliance officers.
Conclusion
Understanding the CMC pharma meaning is crucial for anyone involved in the pharmaceutical industry. It encapsulates the essential processes of Chemistry, Manufacturing, and Controls, which are vital for the development and production of safe and effective medications. This concept not only serves as a regulatory framework but also plays a pivotal role in ensuring that pharmaceutical products meet stringent quality standards throughout their lifecycle.
The article highlights several key aspects of CMC, including:
- Its historical evolution
- The integration of Good Manufacturing Practices (GMP)
- The importance of comprehensive documentation
By examining case studies, such as those involving AVS Life Sciences, it becomes evident that a robust CMC strategy is integral to navigating regulatory complexities, enhancing product quality, and ensuring timely approvals. The emphasis on quality control measures and the need for thorough testing further reinforce the significance of CMC in maintaining compliance and safeguarding patient safety.
As the pharmaceutical landscape continues to evolve, the importance of understanding CMC cannot be overstated. Stakeholders are encouraged to prioritize the implementation of effective CMC strategies to not only comply with regulations but also to foster innovation in drug development. By doing so, they can contribute to the successful delivery of new therapies that ultimately improve patient outcomes and advance public health.
Frequently Asked Questions
What does CMC mean in pharmaceuticals?
CMC stands for Chemistry, Manufacturing, and Controls, which refers to the processes integral to medication development and production, including the substance's chemical composition, manufacturing processes, control measures for standards, and stability assessments.
Why is CMC important in the pharmaceutical industry?
CMC is critical for ensuring that pharmaceutical products are consistently produced and controlled according to stringent quality standards, impacting patient safety and medication efficacy throughout the product lifecycle.
What recent advancements have been made in CMC regulations?
The FDA launched the Chemistry, Manufacturing, and Controls Development and Readiness Pilot (CDRP) Program in 2022 to accelerate CMC development for investigational new drug (IND) applications, promoting enhanced communication between the FDA and sponsors for earlier patient access to innovative therapies.
How is the biologics market related to CMC?
The biologics market is expected to grow at a compound annual growth rate of 15% until 2027, making the understanding of CMC increasingly critical, especially for complex biologics such as monoclonal antibodies.
What role does AVS Life Sciences play in CMC?
AVS Life Sciences is known for delivering rapid value creation and meticulous project execution in CMC, achieving an 80% repeat business rate. They provide expertise in CMC through comprehensive GMP audits, helping clients navigate compliance intricacies.
Can you provide an example of AVS Life Sciences' work in CMC?
A recent project demonstrated how AVS Life Sciences assisted a client in optimizing their CMC processes, resulting in on-time project delivery and zero findings during audits.
What is the overall significance of understanding CMC in pharmaceuticals?
Understanding CMC is vital not only for securing regulatory approval but also for safeguarding patient safety and ensuring the efficacy of medications as the pharmaceutical landscape evolves.
