Understanding Biotechnology Consulting: Importance and Key Features

Overview
Biotechnology consulting plays a pivotal role for organizations within the life sciences sector, offering specialized guidance on:
- Regulatory compliance
- GMP Quality Engineering
- Operational efficiency
This support is essential for the successful development and commercialization of biotechnological products. As the complexities of regulatory frameworks intensify, the demand for these consulting services is on the rise, driven by the anticipated growth of the life sciences market.
Real-world examples illustrate how consulting has significantly enhanced product development processes and outcomes, underscoring the value of expert guidance in navigating compliance challenges and health regulations. By engaging with AVS Life Sciences, organizations can leverage these insights to streamline their operations and improve market readiness.
Introduction
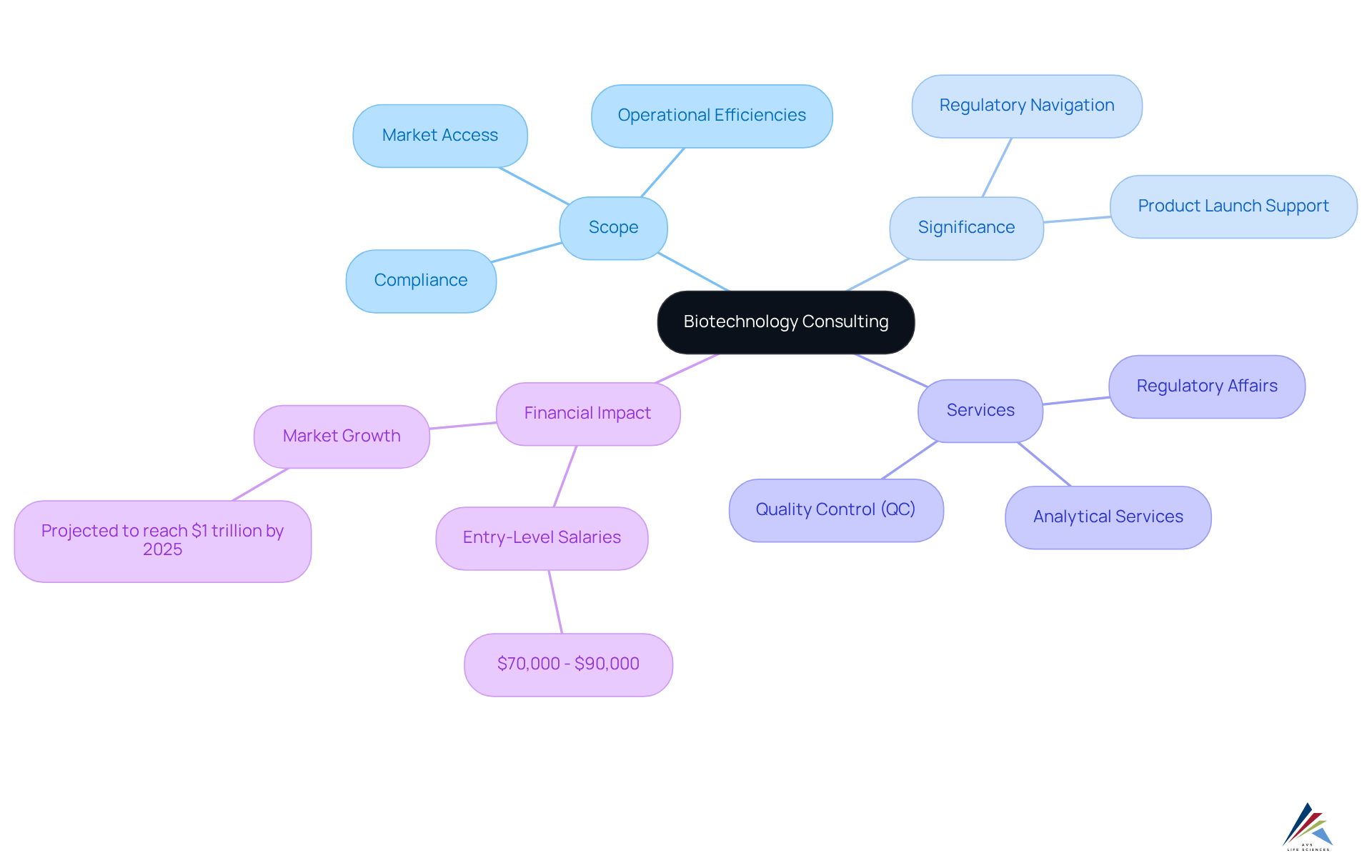
Biotechnology consulting stands at the intersection of innovation and regulation, guiding organizations through the intricate maze of compliance, market entry and commercial manufacturing. As the life sciences sector rapidly expands, projected to near $1 trillion by 2025, the demand for specialized consulting services has never been more critical.
Organizations face significant challenges in harnessing these consulting services effectively. They must navigate complex regulatory landscapes while striving for market entry and compliance. To overcome these hurdles, leveraging expert support becomes paramount. By engaging with seasoned consultants, organizations can adopt tailored strategies that address their unique compliance needs. This proactive approach not only mitigates risks but also positions them for successful market integration.
As we delve deeper into the intricacies of biotechnology consulting, it is essential to explore how these expert partnerships can transform compliance challenges into opportunities for growth and innovation.
Defining Biotechnology Consulting: Scope and Importance
Biotechnology consulting services provide specialized guidance tailored for organizations engaged in the research, development, and commercialization of biotechnological products. This field encompasses a wide range of activities, including compliance with regulations and operational efficiencies. The significance of life sciences consulting is underscored by its ability to assist companies in navigating the complex landscape of regulations and scientific advancements, enabling them to effectively launch innovative products to the market while adhering to industry standards.
As the life sciences sector is projected to experience substantial growth, with the market anticipated to approach approximately $1 trillion by 2025, the demand for professional advice in overcoming regulatory hurdles and improving operational processes is more critical than ever. Moreover, the significance of biotechnology consulting is highlighted through its contributions to product development. Consultants offer essential support in navigating regulatory submissions, clinical trial protocols and inspection readiness ensuring compliance with both local and international standards. Specific services provided by AVS Life Sciences, such as Analytical, QC, CMC, Validation & Regulatory Affairs, enhance the overall efficiency of the product development lifecycle. This expertise not only mitigates risks associated with delays or penalties but also guarantees reliable test results and adherence to quality assurance requirements.
Expert opinions indicate that the expansion of life science innovations—particularly in genomics, bioengineering, and pharmaceuticals—has escalated the demand for biotechnology consulting services. By partnering with biotech advisors, organizations can adeptly navigate the ever-evolving landscape of life sciences, fostering sustainable growth and innovation while ensuring that safe and effective products are delivered to the market promptly.
Historical Context: The Evolution of Biotechnology Consulting
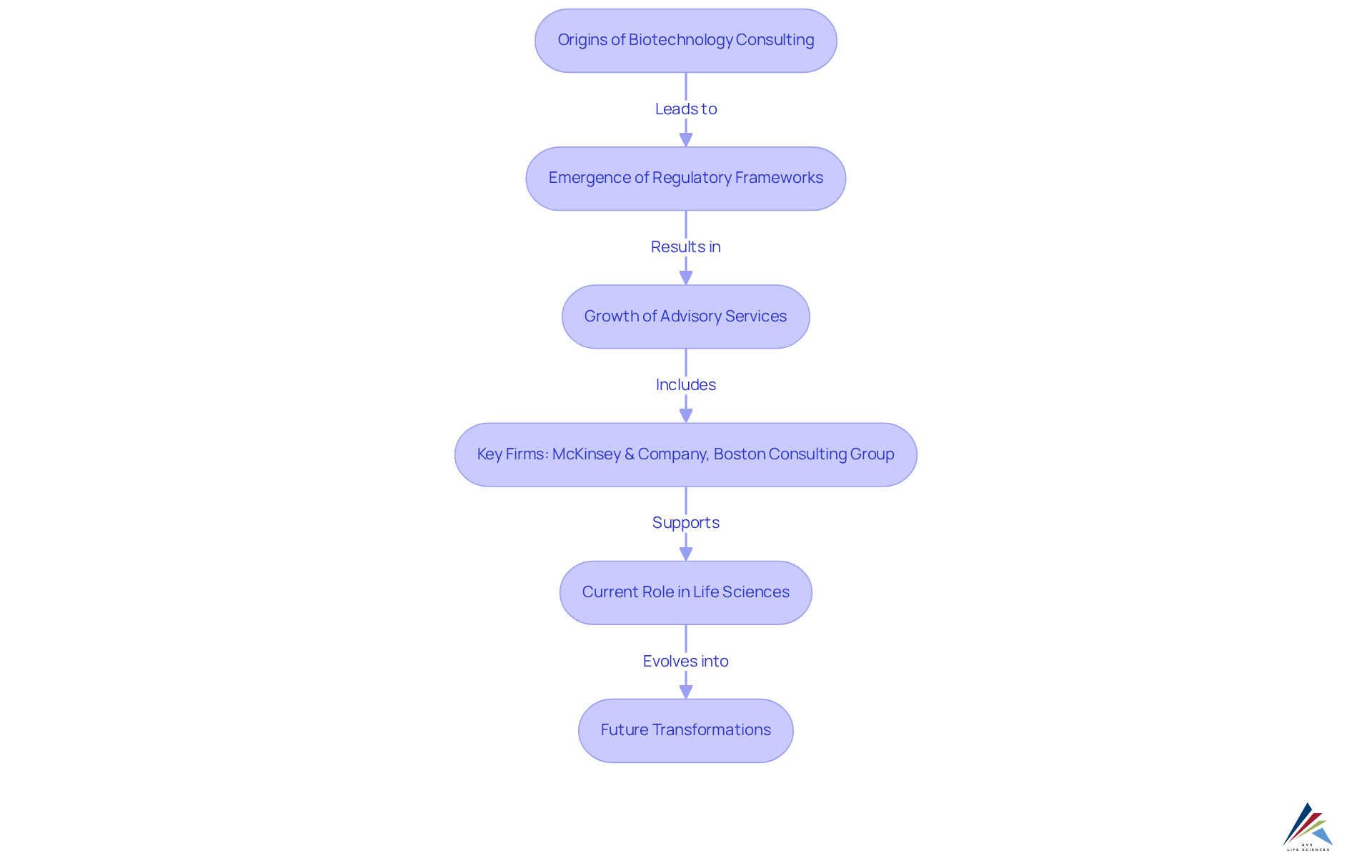
The origins of biotechnology consulting can be traced back to the late 20th century, a time characterized by groundbreaking advancements in genetic engineering and molecular biology. As businesses began to leverage the potential of these advancements, the need for specialized knowledge in compliance and biotechnology consulting for product development became evident. Initially, consultants focused on guiding firms through the emerging regulatory frameworks established by agencies such as the FDA, ensuring adherence to compliance standards.
As the field of biological technology progressed, fueled by innovative technologies and treatments, advisory services grew considerably. This evolution included the incorporation of strategic planning, market analysis, and risk management and quality testing reflecting the industry's increasing complexity. Today, biotechnology consulting plays a vital role in the life sciences ecosystem, offering essential support to organizations throughout every stage of product development. AVS Life Sciences exemplifies this role through its extensive knowledge in validation, quality compliance, and compliance solutions tailored for the life sciences sector. The company provides a variety of services, including engineering support, submissions guidance, and training, all delivered by a worldwide team of over 300 skilled associates.
A notable case study highlights AVS's successful upgrade of a biotechnology client's GMP facility from Level 1 to Level 2 standards, ensuring compliance with critical regulatory frameworks and operational excellence. This project not only met the client's needs but also enhanced their ability to manufacture critical medications, showcasing the transformative impact of effective consulting.
There is widespread excitement for biotechnology's potential to heal illnesses and alleviate pain, highlighting the significance of skilled advisors in biotechnology consulting, such as AVS Life Sciences, in steering biotech companies through compliance challenges and market dynamics. Furthermore, the future of biotech advisory services is poised for significant transformation driven by technological advancements, as highlighted in various case studies. As the sector continues to progress, the significance of skilled advisors in assisting biotech companies through compliance challenges and Good Manufacturing Practices (GMP) requirements remains crucial.
Key Characteristics of Biotechnology Consulting Services
Several essential attributes characterize biotechnology consulting services that address the complex compliance challenges faced by organizations in the life sciences sector.
- Regulatory Expertise: Consultants must possess a profound understanding of regulatory frameworks, including GMP, ISO standards, and Quality System Regulations (QSR). This expertise is vital for guiding clients through compliance challenges, ensuring adherence to stringent industry standards.
- Scientific Knowledge: A robust foundation in biological sciences is essential, as specialists often collaborate with R&D teams to enhance product development processes. This scientific acumen enables them to provide informed insights that drive innovation and efficiency.
- Strategic Insight: Effective consultants deliver strategic guidance that aligns with clients' business objectives, facilitating navigation through market access and competitive positioning. Their ability to analyze market dynamics and compliance landscapes is crucial for formulating effective strategies.
- Risk Management: Identifying and mitigating risks associated with product development and regulatory compliance is a core function of life sciences advisory. By proactively addressing potential challenges, consultants assist clients in avoiding costly setbacks and ensuring smoother project execution.
These attributes underscore the specialized nature of biotechnology consulting services, which necessitates a unique blend of scientific knowledge and business strategy. The increasing complexity of compliance requirements further accentuates the importance of these characteristics, as organizations seek reliable partners to navigate the evolving landscape of the life sciences sector.

Practical Applications: Real-World Examples of Biotechnology Consulting
Biotechnology consulting services have proven essential in driving successful outcomes across the industry. Take, for instance, a biotech startup dedicated to creating an innovative gene therapy. This company engaged biotechnology consulting services to navigate the intricate compliance landscape. The advisors provided comprehensive assistance on regulatory submissions, culminating in the successful approval of their Investigational New Drug (IND) application—a vital milestone in the drug development process.
In another scenario, a major pharmaceutical firm sought the expertise of biotechnology consulting advisors to improve its clinical trial operations. By adopting best practices and streamlining processes, the consultants achieved a notable reduction in trial timelines and costs. This optimization not only improved the company's efficiency but also significantly accelerated its ability to introduce new therapies to the market.
A transformative case study highlighting AVS Life Sciences illustrates their successful upgrade of a biotechnology GMP facility. They assisted a leading San Francisco-based biotech company in transitioning from a Biosafety Level 1 to a Level 2 GMP facility. This project was completed on time and within budget, underscoring AVS's commitment to quality assurance and adherence to standards. Their thorough documentation efforts were recognized by the client’s quality assurance team, ensuring full traceability throughout the process. During this transition, significant lessons emerged regarding anomalies in test results due to improperly installed barcode scanner cameras. This prompted the QC laboratory team to evaluate their business processes and identify gaps that led to unreliable results. This partnership enabled the client to focus on developing medications that enhance patient quality of life, while AVS's expertise in biotechnology consulting and quality management proved essential.
These case studies highlight the varied applications of biotechnology consulting, demonstrating its crucial role in fostering innovation and ensuring adherence in a sector characterized by rapid transformation.
Conclusion
Biotechnology consulting stands at the forefront of innovation in the life sciences sector, playing a crucial role in guiding organizations through the complexities of product development and regulatory compliance. By leveraging specialized knowledge and strategic insights, consultants empower biotech companies to navigate an increasingly intricate landscape, ultimately facilitating the successful introduction of groundbreaking products to the market.
Throughout the article, key themes emerged, including:
- The historical evolution of biotechnology consulting
- The essential characteristics that define effective consulting services
- Real-world applications that illustrate its impact
The narrative highlighted how consultants provide invaluable support in:
- Regulatory expertise
- Commissioning/Qualification and Validation
- Scientific knowledge
- Strategic insight
- Risk management
All of which are vital for fostering innovation and ensuring compliance in a rapidly changing environment. Case studies showcased the tangible benefits of engaging biotech advisors, underscoring their role in enhancing operational efficiencies and driving successful outcomes.
Frequently Asked Questions
What is biotechnology consulting?
Biotechnology consulting involves specialized guidance for organizations engaged in the research, development, and commercialization of biotechnological products, covering areas such as regulatory compliance, market access strategies, and operational efficiencies.
Why is biotechnology consulting important?
It helps companies navigate complex regulations and scientific advancements, enabling them to launch innovative products while adhering to industry standards. This is increasingly critical as the life sciences sector is projected to grow significantly.
What services do biotechnology consultants provide?
Consultants offer support in regulatory submissions, clinical trial protocols, market strategies, GMP Support and Qualification and Validation, among others, to enhance the product development lifecycle.
How does biotechnology consulting impact product development?
It ensures compliance with local and international standards, mitigates risks associated with delays or penalties, and guarantees reliable and reproducible test results and adherence to quality assurance standards.
How can organizations benefit from partnering with biotech advisors?
By collaborating with biotech advisors, organizations can navigate the evolving landscape of life sciences, fostering sustainable growth and innovation while ensuring timely delivery of high-quality products to the market.
