Top Validation Tools Reviews from Gartner for Life Sciences Compliance

Overview
The article underscores the critical role of validation tools in ensuring compliance within the life sciences sector, as emphasized in reviews from Gartner. These tools are not merely beneficial; they are indispensable for adhering to regulatory standards, reducing human error, and improving operational efficiency. Their capacity to provide documented proof of compliance is paramount, enabling organizations to navigate and adapt to the ever-evolving landscape of regulations effectively.
Introduction
The life sciences sector faces relentless scrutiny as it navigates stringent regulatory standards that govern product quality and safety. In this high-stakes landscape, validation tools stand out as essential allies, automating compliance processes and boosting operational efficiency. Yet, with a multitude of options at their disposal, how can organizations identify which validation solutions genuinely fulfill their requirements and ensure compliance with evolving regulations? This article explores the latest reviews from Gartner, comparing leading validation tools and providing insights that empower life sciences companies to effectively tackle compliance challenges.
Significance of Validation Tools in Life Sciences Compliance
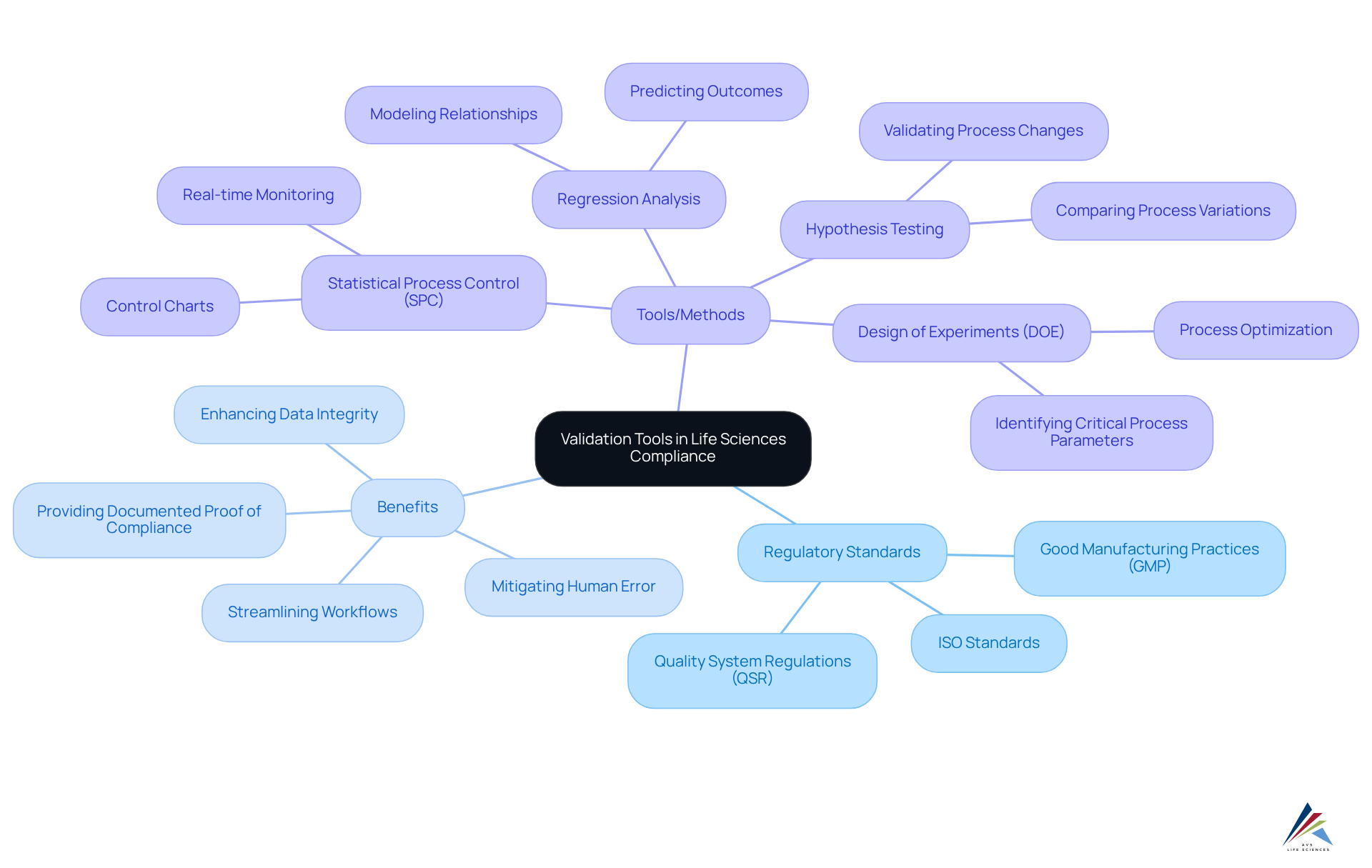
According to , validation instruments play a pivotal role in the , ensuring that procedures, systems, and products meet stringent such as , ISO, and . By automating verification processes, these tools significantly mitigate the risk of human error, enhance data integrity, and streamline workflows. They also furnish essential , which is critical during audits and inspections. Recent advancements in validation tools reviews (Gartner) have further solidified their importance in maintaining standards, enabling organizations to swiftly adapt to changing regulatory environments while ensuring product safety and efficacy.
For instance, the integration of facilitates real-time monitoring of critical quality attributes, ensuring that manufacturing operations remain within specified limits. This proactive strategy not only bolsters compliance but also cultivates operational excellence. Moreover, the application of has proven invaluable for process optimization, empowering manufacturers to pinpoint critical process parameters and their optimal settings, thus guaranteeing consistent product quality.
Real-world examples underscore the effectiveness of these assessment instruments. Organizations that have embraced robust verification strategies report improved , with many achieving zero issues during regulatory inspections. As the life sciences industry continues to evolve, the importance of highlighted in validation tools reviews (Gartner) in ensuring adherence to GMP, ISO, and QSR standards cannot be overstated.
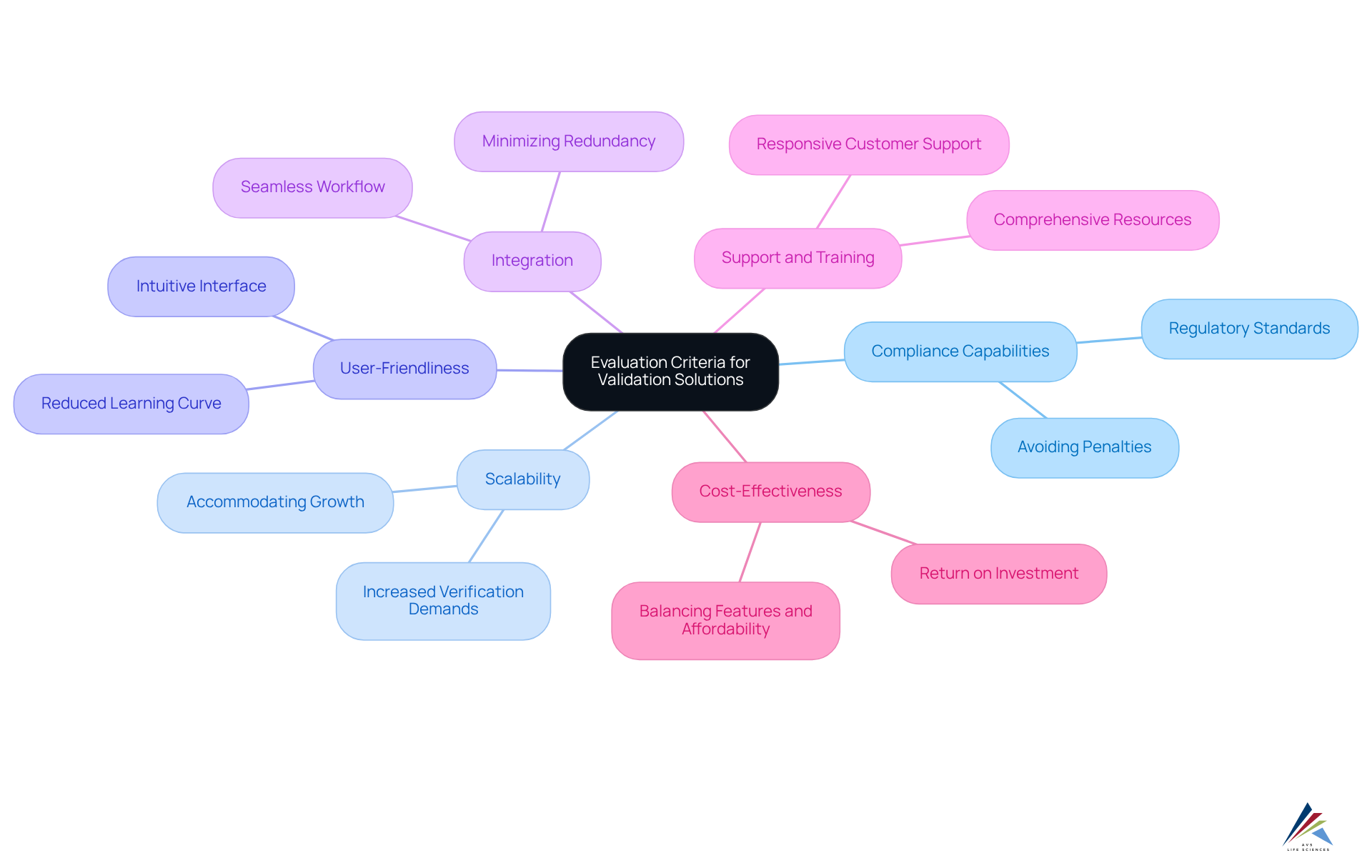
Evaluation Criteria for Comparing Validation Solutions
When evaluating validation solutions, organizations must consider several critical criteria to ensure compliance and operational efficiency:
- : The tool must align with essential regulatory standards, including , ISO standards, and , as well as GXP and FDA regulations. This alignment is crucial for maintaining and avoiding costly penalties.
- Scalability: The solution should accommodate organizational growth, allowing for increased verification demands without a corresponding rise in manual effort. This flexibility is vital as companies expand their operations and product lines.
- User-Friendliness: An intuitive interface is essential for ensuring that users can navigate the system effectively without extensive training. A enhances productivity and reduces the learning curve for new users.
- Integration: The ability to seamlessly integrate with existing systems and workflows is critical for enhancing efficiency and minimizing redundancy. Effective integration ensures that validation processes are streamlined and data flows smoothly across platforms.
- Support and Training: Comprehensive support and training resources are essential for maximizing the effectiveness of the system. Organizations should prioritize solutions that offer robust training programs and responsive customer support to address any challenges that arise.
- Cost-Effectiveness: The solution should deliver a strong return on investment, balancing features with affordability. Organizations must evaluate the long-term worth of the resource in relation to its cost, ensuring that it fulfills both regulatory and budgetary requirements.
By methodically assessing (gartner) against these standards, organizations can make knowledgeable choices that correspond with their regulatory aims and operational objectives. A recent case study emphasized how a pharmaceutical firm enhanced its compliance abilities by implementing a verification tool that satisfied all these criteria, leading to a notable decrease in audit findings and improved product quality. This highlights the significance of choosing the appropriate assessment solution in today’s regulatory environment.
Comparison of Leading Validation Tools from Gartner
Based on the latest reviews from Gartner, a comparison of three leading validation tools reveals distinct advantages tailored for organizations navigating :
-
ValGenesis VLMS:
- : Demonstrates strong alignment with GMP and ISO standards, ensuring .
- Scalability: Highly scalable, making it suitable for organizations of all sizes, from startups to large enterprises.
- User-Friendliness: Features an intuitive interface complemented by robust training resources, facilitating user adoption.
- Integration: Offers seamless integration with existing systems, minimizing disruption during implementation.
- Support and Training: and comprehensive , enhancing user experience.
- Cost-Effectiveness: While it requires a higher initial investment, it promises long-term savings through efficiency gains.
-
Kneat Gx:
- Compliance Capabilities: Incorporates comprehensive specifically tailored for the , addressing unique regulatory needs.
- Scalability: Designed to grow alongside the organization, adapting to evolving requirements.
- User-Friendliness: Boasts a ; however, some users report a learning curve that may require additional support.
- Integration: Exhibits good integration capabilities with other software, ensuring compatibility within diverse tech ecosystems.
- Support and Training: Features a strong support network, although training materials could be more extensive to aid user onboarding.
- Cost-Effectiveness: Offers competitive pricing with a focus on delivering value, appealing to budget-conscious organizations.
-
MasterControl:
- Compliance Capabilities: Excels in compliance tracking and reporting features, providing organizations with critical oversight.
- Scalability: Scalable for both small and large enterprises, making it versatile across various operational scales.
- User-Friendliness: Generally user-friendly, though some advanced features may necessitate additional training for optimal use.
- Integration: Integrates well with various enterprise systems, promoting operational efficiency.
- Support and Training: Offers comprehensive support and training options, ensuring users are well-equipped to utilize the tool effectively.
- Cost-Effectiveness: Positioned at mid-range pricing, it delivers a strong return on investment (ROI).
This comparison emphasizes the distinct advantages of each instrument, as outlined in (Gartner), empowering organizations to select the solution that most appropriately meets their particular requirements.

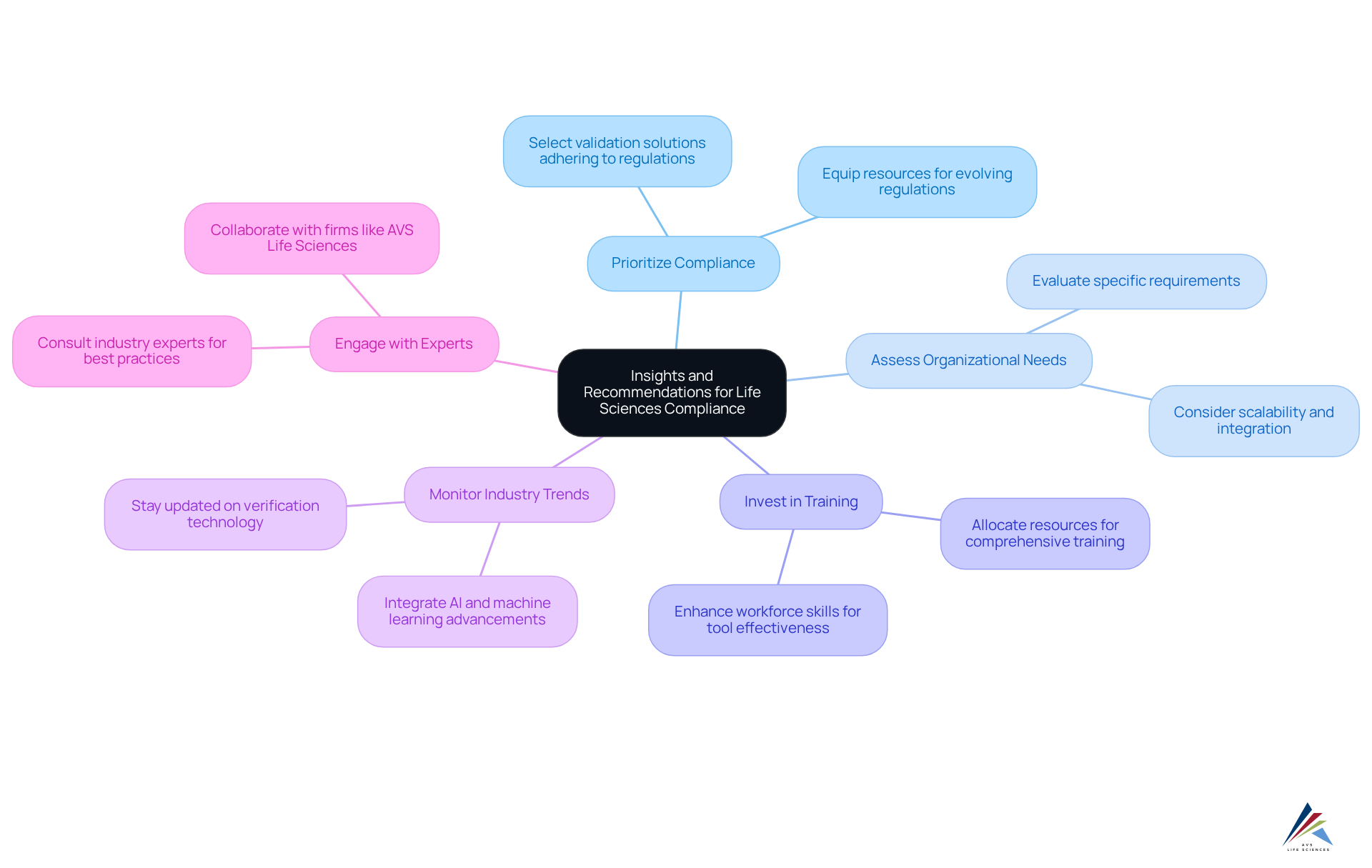
Insights and Recommendations for Life Sciences Compliance
In the realm of (Gartner) for the life sciences sector, organizations face significant that necessitate strategic solutions. To address these issues effectively, several key insights and recommendations emerge:
- Prioritize Compliance: Selecting a validation solution that adheres to the latest is crucial to avert future compliance issues. Organizations must ensure that their resources are equipped to meet evolving regulations, thereby safeguarding their operations.
- Assess Organizational Needs: A thorough evaluation of your organization’s specific requirements, including scalability and integration capabilities, is essential. By selecting an instrument that aligns with these needs, organizations can facilitate growth and adaptability in a rapidly changing environment.
- Invest in Training: Allocating resources for is vital to maximize the effectiveness of the chosen validation instrument. A skilled workforce can leverage the features of the resource, leading to enhanced adherence results. Research indicates that companies with robust training programs experience a notable improvement in tool efficiency, significantly boosting overall adherence.
- Monitor Industry Trends: Staying abreast of , such as the integration of AI and machine learning, is essential. These advancements can streamline verification methods and fortify adherence initiatives, rendering them more efficient and responsive to .
- Engage with Experts: Consulting with or firms like can yield valuable insights into best practices. Such collaboration ensures that verification methods are not only compliant but also robust and efficient.
By adhering to these recommendations, organizations can fortify their compliance frameworks and optimize their , as noted in validation tools reviews (Gartner), ultimately leading to improved regulatory outcomes. Engage with AVS Life Sciences to explore tailored solutions that meet your compliance needs.
Conclusion
The role of validation tools in ensuring compliance within the life sciences sector is paramount. These instruments not only help organizations meet regulatory standards but also enhance operational efficiency and data integrity. By automating verification processes, they significantly reduce the risk of human error, providing a solid foundation for compliance with frameworks such as GMP, ISO, and QSR.
Essential criteria for selecting validation solutions include:
- Compliance capabilities
- Scalability
- User-friendliness
- Integration
- Support
- Cost-effectiveness
Each of these factors plays a crucial role in determining the effectiveness of a validation tool in addressing the unique challenges faced by life sciences organizations. Furthermore, the comparison of leading tools from Gartner—ValGenesis VLMS, Kneat Gx, and MasterControl—demonstrates that while each offers distinct advantages, the right choice ultimately depends on an organization’s specific needs and operational context.
In light of the evolving regulatory landscape, it is vital for organizations to prioritize compliance and invest in the right validation tools that align with their goals. By staying informed about industry trends and engaging with experts, organizations can enhance their compliance frameworks. This proactive approach not only improves regulatory outcomes but also positions companies for long-term success in a competitive market. Embracing these recommendations will empower organizations to navigate the complexities of life sciences compliance effectively.
Frequently Asked Questions
What is the significance of validation tools in the life sciences sector?
Validation tools are crucial in the life sciences sector as they ensure that procedures, systems, and products comply with regulatory standards such as Good Manufacturing Practices (GMP), ISO, and Quality System Regulations (QSR).
How do validation tools enhance data integrity?
By automating verification processes, validation tools significantly reduce the risk of human error, which enhances data integrity and streamlines workflows.
Why is documented proof of compliance important?
Documented proof of compliance is essential during audits and inspections, as it provides evidence that organizations meet regulatory requirements.
What advancements have been made in validation tools?
Recent advancements in validation tools have strengthened their role in maintaining compliance and have enabled organizations to adapt quickly to changing regulatory environments while ensuring product safety and efficacy.
How do statistical process control (SPC) tools contribute to compliance?
SPC tools facilitate real-time monitoring of critical quality attributes, ensuring that manufacturing operations stay within specified limits, which bolsters compliance and promotes operational excellence.
What role does Design of Experiments (DOE) play in process optimization?
DOE is invaluable for process optimization, allowing manufacturers to identify critical process parameters and their optimal settings, thus ensuring consistent product quality.
Can you provide examples of the effectiveness of validation tools?
Organizations that have implemented robust verification strategies often report improved compliance outcomes, with many achieving zero issues during regulatory inspections.
Why is the importance of advanced verification instruments emphasized in the article?
The article emphasizes the importance of advanced verification instruments in ensuring adherence to GMP, ISO, and QSR standards, especially as the life sciences industry continues to evolve.
