Navigate the Cosmetic Supply Chain Transparency Act for Compliance Success

Overview
The Cosmetic Supply Chain Transparency Act is designed to enhance compliance by mandating that suppliers provide comprehensive information regarding the raw materials and ingredients utilized in cosmetics. This initiative promotes safety and transparency within the industry.
To navigate the complexities of the Act effectively, companies must adopt essential strategies for compliance. These strategies include:
- Fostering effective communication with suppliers
- Implementing meticulous documentation practices
- Conducting thorough staff training
- Performing regular audits
Collectively, these measures not only facilitate adherence to the Act but also play a crucial role in building consumer trust. By embracing these practices, businesses can position themselves as leaders in compliance and safety, ultimately driving engagement with AVS Life Sciences.
Introduction
The increasing focus on consumer safety has catalyzed the introduction of the Cosmetic Supply Chain Transparency Act, a crucial piece of legislation that mandates thorough disclosure from suppliers. This act not only seeks to enhance transparency within the cosmetic industry but also addresses the pressing need for safer beauty products by regulating the inclusion of harmful chemicals.
As companies navigate the complexities of compliance, they encounter the challenge of grasping intricate requirements and implementing effective strategies.
How can organizations ensure they meet these new standards while sustaining operational efficiency and consumer trust?
Understand the Cosmetic Supply Chain Transparency Act
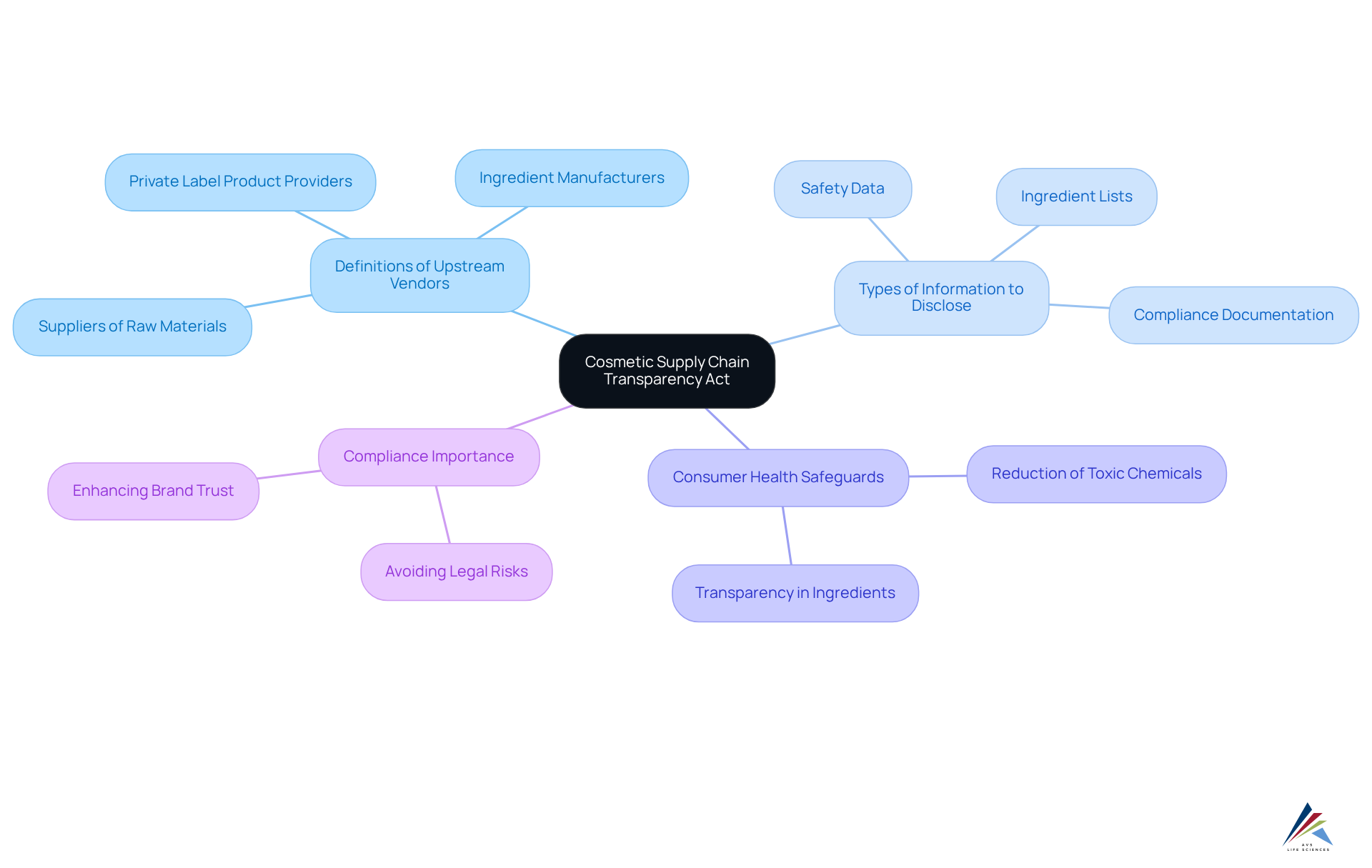
The Cosmetic Supply Chain Transparency Act requires that suppliers of raw materials, ingredients, and private label products provide detailed information about their offerings to cosmetic companies. This requirement encompasses , designed to bolster transparency and safeguard consumer health. As part of a larger initiative promoting safer beauty products, the Cosmetic Supply Chain Transparency Act addresses pressing concerns regarding the presence of toxic chemicals in cosmetics. Familiarizing oneself with these requirements is essential for achieving compliance and protecting public health. It is crucial to understand the key elements of the Cosmetic Supply Chain Transparency Act, which includes:
- Definitions of upstream vendors
- The specific types of information that must be disclosed
Identify Compliance Requirements Under the Act
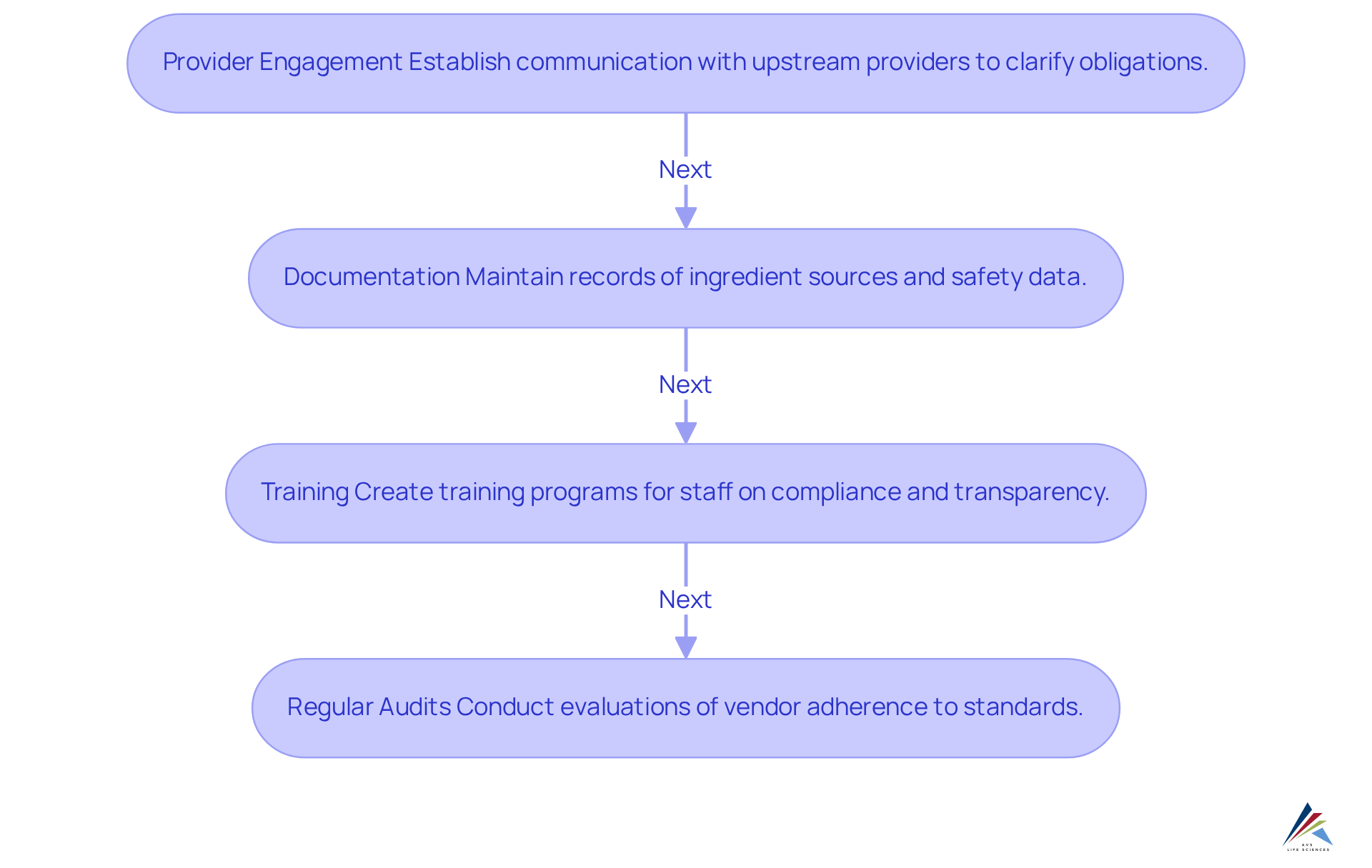
To comply with the Cosmetic Supply Chain Transparency Act, companies must implement several essential strategies:
- Provider Engagement: Establish robust communication channels with all upstream providers to clarify their obligations under the Act. This includes obtaining comprehensive , which are critical for ensuring product safety and compliance. AVS Life Sciences provides expert consulting to assist companies in managing these complexities, ensuring that all supplier interactions align with compliance expectations.
- Documentation: Maintain meticulous records of all ingredient sources and their associated safety data. Accurate documentation is vital for audits and regulatory inspections, as it demonstrates adherence to safety standards and regulatory requirements. AVS Life Sciences offers comprehensive quality management solutions to assist companies in maintaining these critical records, including the provision of certificates of analysis and contaminant testing reports.
- Training: Create and execute training programs for personnel engaged in regulatory efforts. Ensuring that staff are knowledgeable about the Act's stipulations and optimal methods for upholding transparency is vital for efficient management. AVS Life Sciences can assist organizations in developing customized training programs that enhance staff knowledge and adherence skills.
- Regular Audits: Conduct systematic evaluations of vendor adherence to verify that all necessary information is being provided and meets the standards established by the Act. Frequent evaluations help identify deficiencies in adherence and promote a culture of responsibility among vendors. AVS Life Sciences specializes in performing GMP evaluations and adherence assessments, ensuring that companies meet the highest standards in the life sciences sector.
By effectively engaging suppliers and maintaining thorough documentation, companies can navigate the complexities of the cosmetic supply chain transparency act, ensuring adherence and enhancing consumer trust in their products.
Implement Changes for Compliance Success
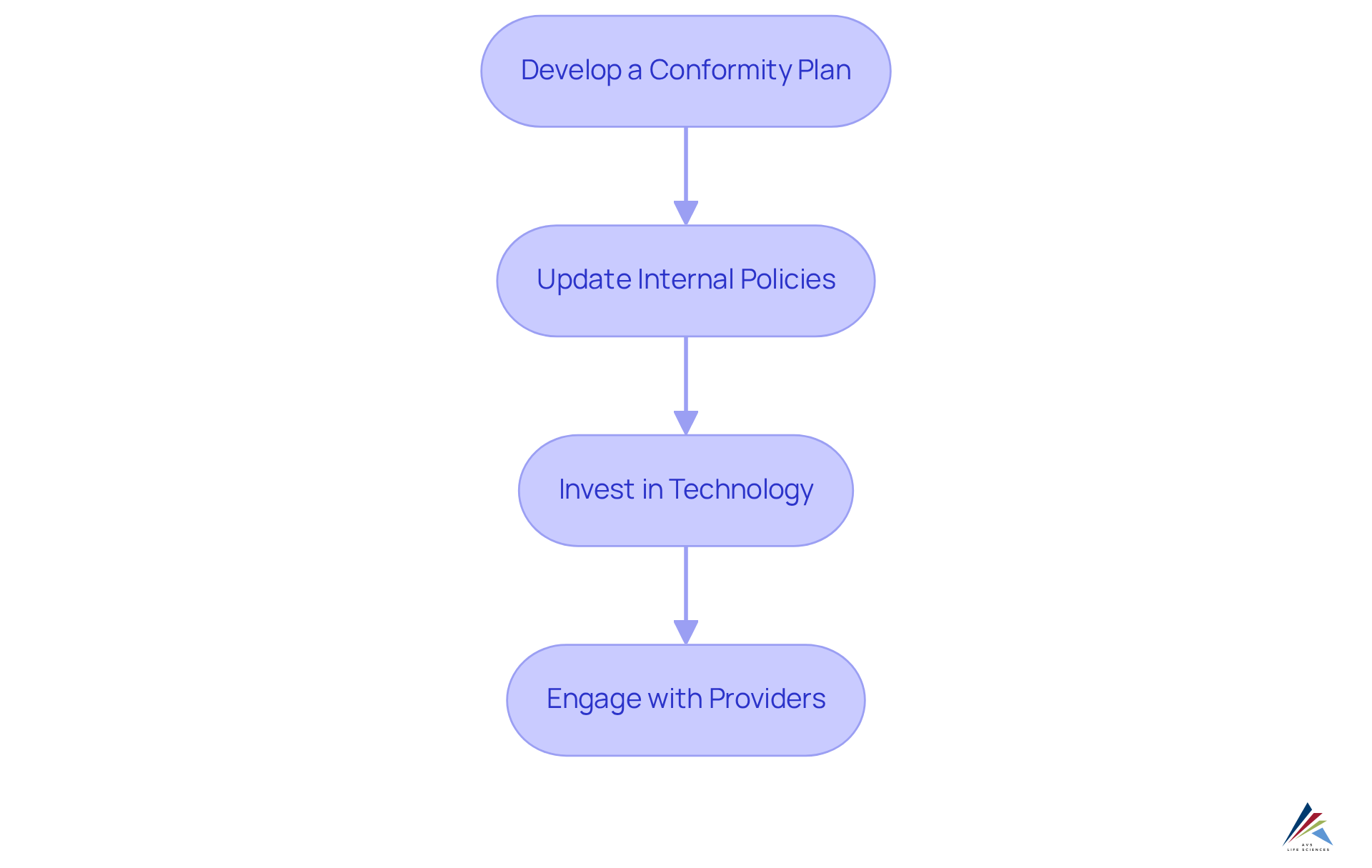
To effectively implement changes for compliance with the Cosmetic Supply Chain Transparency Act, consider the following steps:
- Develop a Conformity Plan: Formulate a comprehensive adherence plan detailing the actions your company will undertake to fulfill the Act's requirements. This plan should include timelines, designated responsibilities, and specific actions to be taken.
- Update Internal Policies: Revise your internal policies and procedures to align with the new regulatory mandates. It is essential that all staff are made aware of these changes and comprehend their duties in maintaining regulatory standards.
- Invest in Technology: Explore management software that can assist in tracking vendor information, managing documentation, and streamlining reporting processes. Technology plays a crucial role in improving efficiency and precision.
- Engage with Providers: Build strong connections with providers to ensure their dedication to transparency and adherence. Regularly assess their practices and provide constructive feedback to help them align with the Act's requirements.
Industry specialists highlight that revising internal policies in accordance with the Cosmetic Supply Chain Transparency Act is not merely a compliance requirement but a strategic action that can improve operational efficiency and stakeholder confidence. Firms such as AVS Life Sciences, which have effectively handled regulatory updates, frequently report enhanced supplier connections and streamlined procedures. This demonstrates the concrete advantages of . Their experience in enhancing GMP facilities underscores the significance of quality assurance and regulatory adherence, illustrating how such initiatives can result in substantial advancements in operational practices. Moreover, utilizing technology in regulatory efforts can greatly diminish the risk of non-adherence and promote a culture of accountability within organizations.
Troubleshoot Common Compliance Challenges
Navigating compliance challenges under the Cosmetic Supply Chain Transparency Act necessitates a proactive approach to several key areas:
- : The non-adherence of vendors can significantly hinder compliance initiatives. Establishing a robust communication protocol is essential for addressing these issues promptly. Implementing penalties for non-compliance serves as a strong incentive for suppliers to adhere to requirements, thereby fostering a culture of accountability.
- Documentation Gaps: Regular reviews of documentation practices are crucial to ensure that all necessary information is collected and maintained. Conducting internal audits can assist in identifying and rectifying any gaps in documentation, which in turn enhances adherence to standards and reduces the risk of penalties.
- Staff Training Deficiencies: Adequate training for all personnel involved in compliance is essential. Scheduling regular training sessions keeps staff informed about the latest regulatory changes and best practices. This continuous education mitigates risks associated with non-adherence and ensures that the team is well-prepared to confront regulatory challenges.
- Regulatory Changes: Staying informed about updates to the cosmetic supply chain transparency act and its related regulations is critical for compliance success. Subscribing to industry newsletters and joining professional organizations provides timely updates and guidance, enabling organizations to adapt swiftly to regulatory changes.
By effectively addressing these common compliance challenges, organizations can enhance their compliance posture and navigate the complexities of the cosmetic supply chain with greater confidence.

Conclusion
Navigating the Cosmetic Supply Chain Transparency Act is not merely a regulatory obligation; it is a crucial step for companies committed to compliance and consumer safety. This Act requires suppliers to furnish comprehensive product information, cultivating a culture of transparency that is indispensable in today's cosmetic industry. By grasping and executing the Act's requirements, businesses can safeguard public health while simultaneously enhancing their operational integrity.
To achieve compliance effectively, companies must:
- Engage with suppliers
- Maintain meticulous documentation
- Provide thorough staff training
- Conduct regular audits
Each of these strategies is integral to establishing a robust compliance framework that can address potential challenges such as vendor non-adherence and documentation gaps. Proactively managing these elements enables companies to cultivate trust with both consumers and stakeholders.
Ultimately, the Cosmetic Supply Chain Transparency Act offers businesses a unique opportunity to refine their practices and reaffirm their dedication to safety and transparency. Embracing these regulatory changes not only mitigates the risks associated with non-compliance but also positions companies as frontrunners in the quest for safer beauty products. As the industry continues to evolve, remaining informed and adaptable will be essential for success in this new regulatory landscape.
Frequently Asked Questions
What is the Cosmetic Supply Chain Transparency Act?
The Cosmetic Supply Chain Transparency Act requires suppliers of raw materials, ingredients, and private label products to provide detailed information about their offerings to cosmetic companies, including safety data and ingredient lists.
What is the purpose of the Cosmetic Supply Chain Transparency Act?
The purpose of the Act is to enhance transparency in the cosmetic supply chain and to safeguard consumer health by addressing concerns regarding toxic chemicals in cosmetics.
What types of information must suppliers disclose under the Act?
Suppliers must disclose detailed information about their offerings, which includes safety data and ingredient lists.
Why is it important to understand the Cosmetic Supply Chain Transparency Act?
Understanding the Act is essential for achieving compliance with its requirements and for protecting public health.
What are upstream vendors in the context of the Cosmetic Supply Chain Transparency Act?
Upstream vendors refer to suppliers involved in the earlier stages of the supply chain who provide raw materials and ingredients to cosmetic companies.
