Navigate the Cosmetic Safety Bill Federal: Key Insights for Compliance Officers

Overview
The article delivers crucial insights for compliance officers concerning the federal cosmetic safety bill, underscoring the necessity of comprehending its provisions to ensure product safety and regulatory adherence. It outlines the bill's mandates for safety assessments, ingredient transparency, and incident reporting. These measures not only safeguard consumers but also compel compliance officers to establish comprehensive documentation and communication strategies within their organizations, enabling them to navigate the evolving regulatory landscape effectively.
Introduction
The introduction of the Cosmetic Safety Bill signifies a pivotal transformation in the regulatory landscape of the cosmetics industry, with a pronounced focus on consumer safety and product transparency. Compliance officers are positioned at the forefront of this evolution, responsible for navigating the intricate requirements inherent to the new legislation.
As the industry confronts the implications of these regulations, a critical question arises: how can stakeholders effectively adapt their practices to not only ensure compliance but also cultivate consumer trust in an increasingly scrutinized market?
This inquiry invites a deeper exploration into proactive strategies that can enhance compliance efforts while reinforcing the integrity of consumer relationships.
Explore the Foundations of the Cosmetic Safety Bill
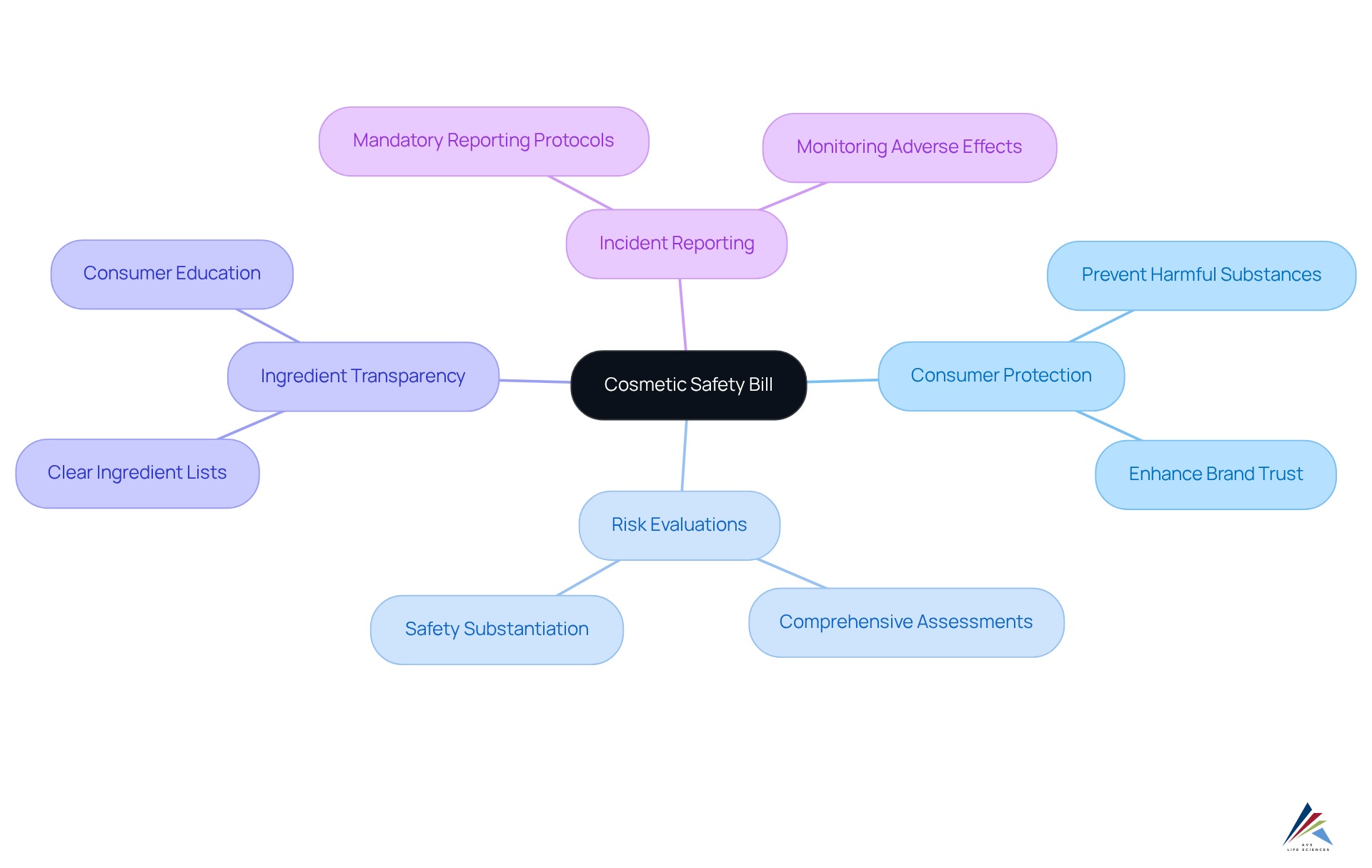
The cosmetic safety bill federal represents a pivotal legislative action designed to bolster consumer protection by ensuring the safety of cosmetic products. This legislation establishes a robust oversight framework that mandates comprehensive risk evaluations, ingredient transparency, and incident reporting. Compliance officers must fully understand the bill's objectives, which aim to prevent harmful substances from entering the market while enforcing stringent protocols among manufacturers.
Regulatory experts emphasize that effective safety assessments are essential in cosmetic product development, as they not only protect consumer health but also foster brand trust. The recent updates to the adherence requirements of the federal cosmetic safety bill further underscore the importance of transparency and accountability within the industry.
By aligning their practices with these regulations, compliance officers can proactively anticipate future changes and play a vital role in creating a safer cosmetic landscape for consumers.
Examine Key Provisions and Regulatory Requirements
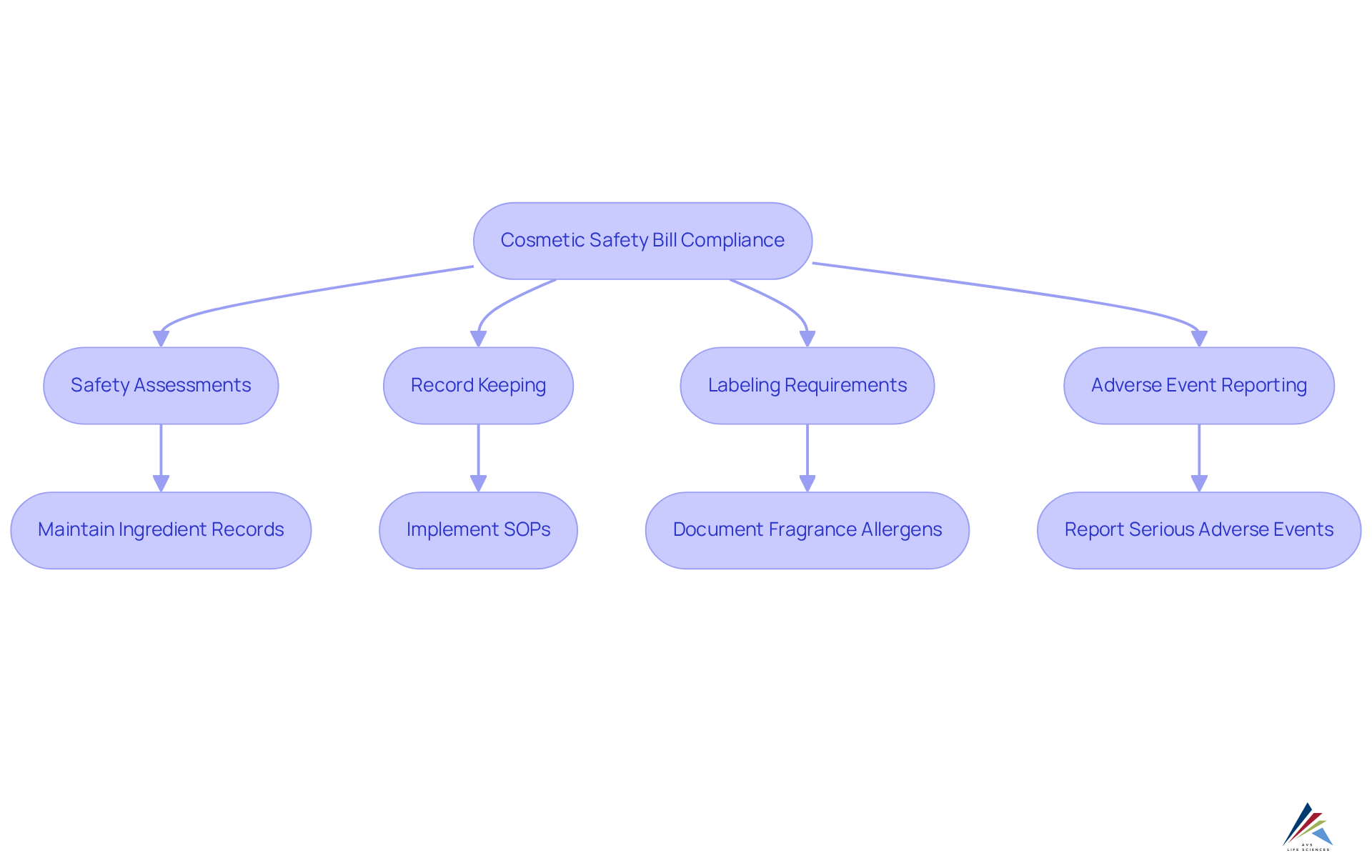
The cosmetic safety bill federal introduces critical provisions mandating safety assessments for all cosmetic products before they enter the market. Manufacturers are required to maintain detailed records of these assessments, including documentation on ingredient sourcing. Furthermore, stringent labeling requirements are enforced, necessitating clear disclosure of all ingredients and potential allergens, which may encompass up to 80 fragrance allergens. A robust system for reporting adverse events is also mandated, ensuring that any serious incidents are promptly communicated to regulatory authorities.
To meet these requirements, oversight personnel must implement thorough documentation practices within their organizations, adhering to GXP standards and FDA regulations. This involves creating and following standard operating procedures (SOPs) for effective record-keeping and evaluation protocols. Specific internal and external auditing methods should be employed to confirm adherence to these provisions, assisting in identifying any regulatory gaps and ensuring that all risk evaluations remain current. Businesses that prioritize these practices not only enhance their compliance status but also bolster consumer protection and confidence in their products. For tailored consulting solutions in quality management and regulatory standards, compliance officers are encouraged to engage with AVS Life Sciences.
Assess the Impact on Industry Stakeholders and Compliance Practices
The cosmetic safety bill federal is set to transform the landscape for stakeholders within the cosmetics industry. Producers are expected to significantly increase their investments in testing and compliance infrastructure to adhere to the cosmetic safety bill federal regulations. This transition is vital as the industry braces for a surge in demand for comprehensive evaluations, with companies projected to allocate substantial resources toward the cosmetic safety bill federal initiatives. Retailers, in response, must ensure that the products they offer align with the updated security standards outlined in the cosmetic safety bill federal, thereby reinforcing their commitment to consumer protection.
Consumers will benefit from improved transparency regarding product ingredients and safety protocols established by the cosmetic safety bill federal, which will cultivate greater trust in the cosmetics they utilize. Compliance officers are urged to proactively assess how the cosmetic safety bill federal and other regulatory changes will affect their organizations and to develop strategies for effectively communicating with all stakeholders. This may include the creation of educational resources aimed at consumers to elucidate the implications of the new regulations, as well as the establishment of extensive training programs for employees to ensure a comprehensive understanding of the regulatory requirements.
Industry leaders underscore the necessity of investing in regulatory infrastructure, especially in light of the cosmetic safety bill federal, with numerous firms reporting a notable increase in their testing budgets for security. For instance, statistics indicate that cosmetic firms are projected to invest significantly in security testing, demonstrating their adherence to the cosmetic safety bill federal and a broader commitment to consumer health and regulatory compliance. As the industry navigates these changes, the focus on the cosmetic safety bill federal will not only enhance product safety but also strengthen brand loyalty and market positioning in an increasingly competitive landscape.

Understand the Legislative Process and Future Prospects
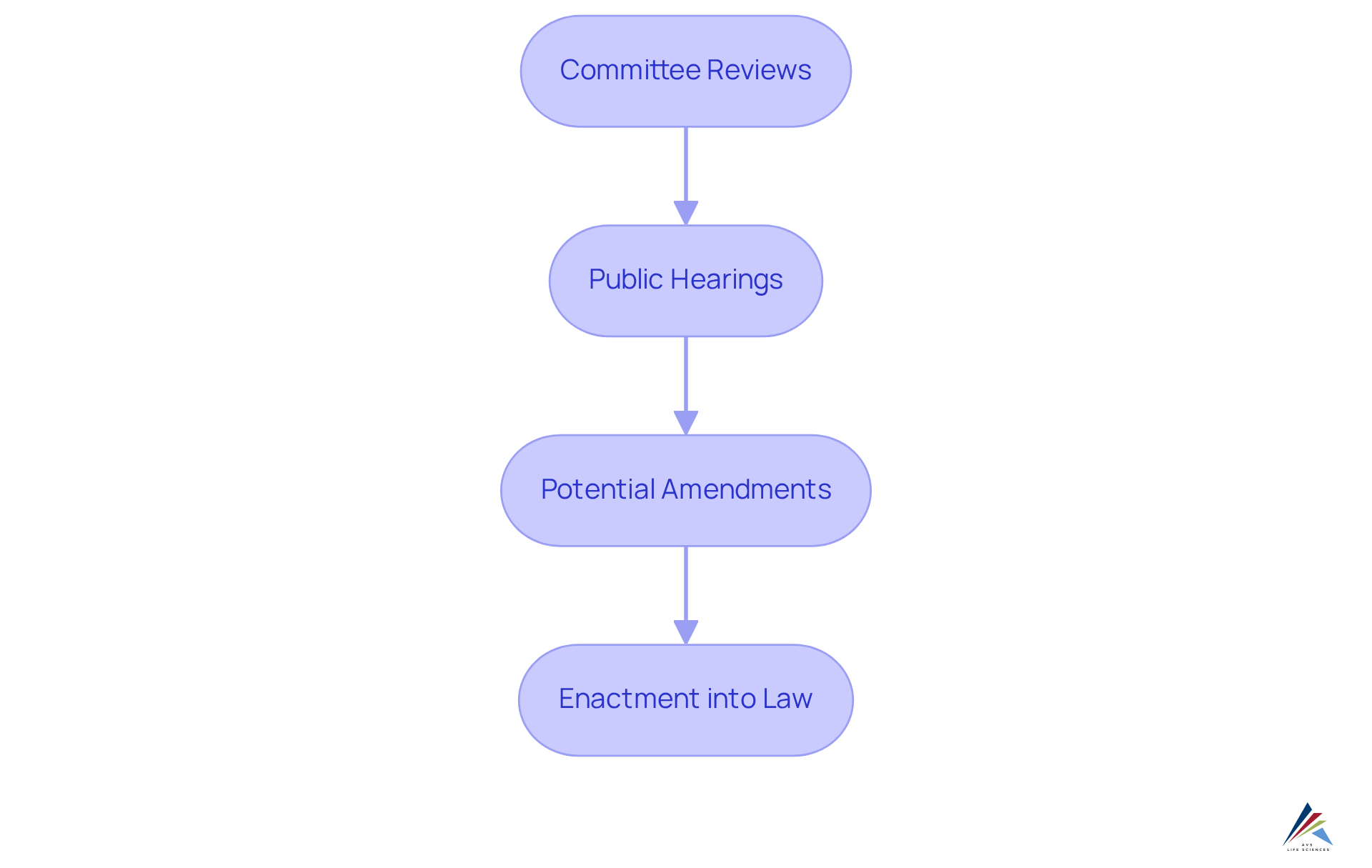
The cosmetic safety bill federal undergoes a legislative journey that involves several critical stages: committee reviews, public hearings, and potential amendments before it can be enacted into law. Compliance professionals must remain vigilant about the progress of the cosmetic safety bill federal and actively engage with industry associations that advocate for their interests. These organizations play a vital role in representing regulatory professionals' perspectives and ensuring their voices are heard in the legislative process. For instance, groups such as the Personal Care Products Council and the Cosmetic Ingredient Review provide essential resources and support for professionals navigating these changes.
Looking ahead, the oversight framework may evolve to include additional rules addressing emerging issues, particularly concerning sustainability and environmental impacts. By closely monitoring these developments, regulatory specialists can prepare their organizations to not only comply with existing guidelines but also adapt swiftly to new demands, thereby maintaining a competitive edge in a shifting regulatory landscape. Furthermore, data underscores the importance of public engagement in the legislative process, as it can significantly sway the outcomes of cosmetic regulations. As Thomas Jefferson aptly stated, when the populace is well-informed, they can be trusted with their own governance. This underscores the necessity for compliance officers to cultivate awareness and advocacy within their organizations.
Conclusion
The federal cosmetic safety bill marks a significant advancement in consumer protection by establishing rigorous safety standards for cosmetic products. Compliance officers must fully grasp its implications, as their role is crucial in ensuring that manufacturers adhere to the updated regulations, ultimately safeguarding consumer health and enhancing brand integrity.
Key provisions of the bill include:
- Mandatory safety assessments
- Stringent labeling requirements
- Robust incident reporting systems
Compliance officers are urged to implement thorough documentation practices and engage in regular audits to ensure adherence to these regulations. This legislation will impact various stakeholders—manufacturers, retailers, and consumers—fostering a culture of transparency and accountability in the cosmetics industry.
As the industry adapts to these new regulations, it is imperative for compliance officers to stay informed and proactive. By investing in regulatory infrastructure and engaging with industry associations, professionals can effectively navigate the evolving landscape of cosmetic safety. The successful implementation of the cosmetic safety bill will not only enhance product safety but also build consumer trust and loyalty, reinforcing the importance of compliance in driving a responsible and sustainable cosmetics industry.
Frequently Asked Questions
What is the purpose of the cosmetic safety bill?
The cosmetic safety bill aims to enhance consumer protection by ensuring the safety of cosmetic products through a robust oversight framework that includes comprehensive risk evaluations, ingredient transparency, and incident reporting.
What are the key components of the cosmetic safety bill?
The key components include risk evaluations of cosmetic products, transparency regarding ingredients, and mandatory incident reporting by manufacturers.
Why is it important for compliance officers to understand the cosmetic safety bill?
Compliance officers need to understand the bill's objectives to prevent harmful substances from entering the market and to enforce stringent protocols among manufacturers, which is crucial for consumer safety.
How do safety assessments impact cosmetic product development?
Effective safety assessments are essential in cosmetic product development as they protect consumer health and foster brand trust.
What recent updates have been made to the federal cosmetic safety bill?
Recent updates have focused on enhancing adherence requirements, emphasizing the importance of transparency and accountability within the cosmetic industry.
How can compliance officers contribute to a safer cosmetic landscape?
By aligning their practices with the regulations of the cosmetic safety bill, compliance officers can anticipate future changes and play a vital role in ensuring consumer safety in the cosmetic industry.
