Mastering GMP Logo Design: A Step-by-Step Guide for Compliance

Overview
This article serves as a comprehensive guide for designing a GMP logo that aligns with Good Manufacturing Practices and pertinent regulatory standards. It underscores the critical importance of clarity, simplicity, and strict adherence to regulations throughout the logo design process. Supported by principles such as effective color choices and meticulous validation steps, the article ensures compliance while enhancing brand credibility within the pharmaceutical sector. By addressing compliance challenges and offering detailed solutions, it provides actionable insights that engage readers and encourage them to embrace best practices in their logo design efforts.
Introduction
Creating a GMP logo transcends mere aesthetics; it embodies a steadfast commitment to quality and compliance, which is crucial in industries where safety is paramount. Good Manufacturing Practices (GMP) are the backbone of ensuring product integrity, making it essential for businesses to grasp the nuances of GMP logo design in order to convey trust and reliability. Yet, with varying regulatory standards and the necessity for clear communication, how can companies ensure their logos not only comply but also resonate with consumers? This guide explores the intricacies of mastering GMP logo design, providing a roadmap for achieving both regulatory adherence and brand recognition.
Understand Good Manufacturing Practices (GMP) and Their Importance
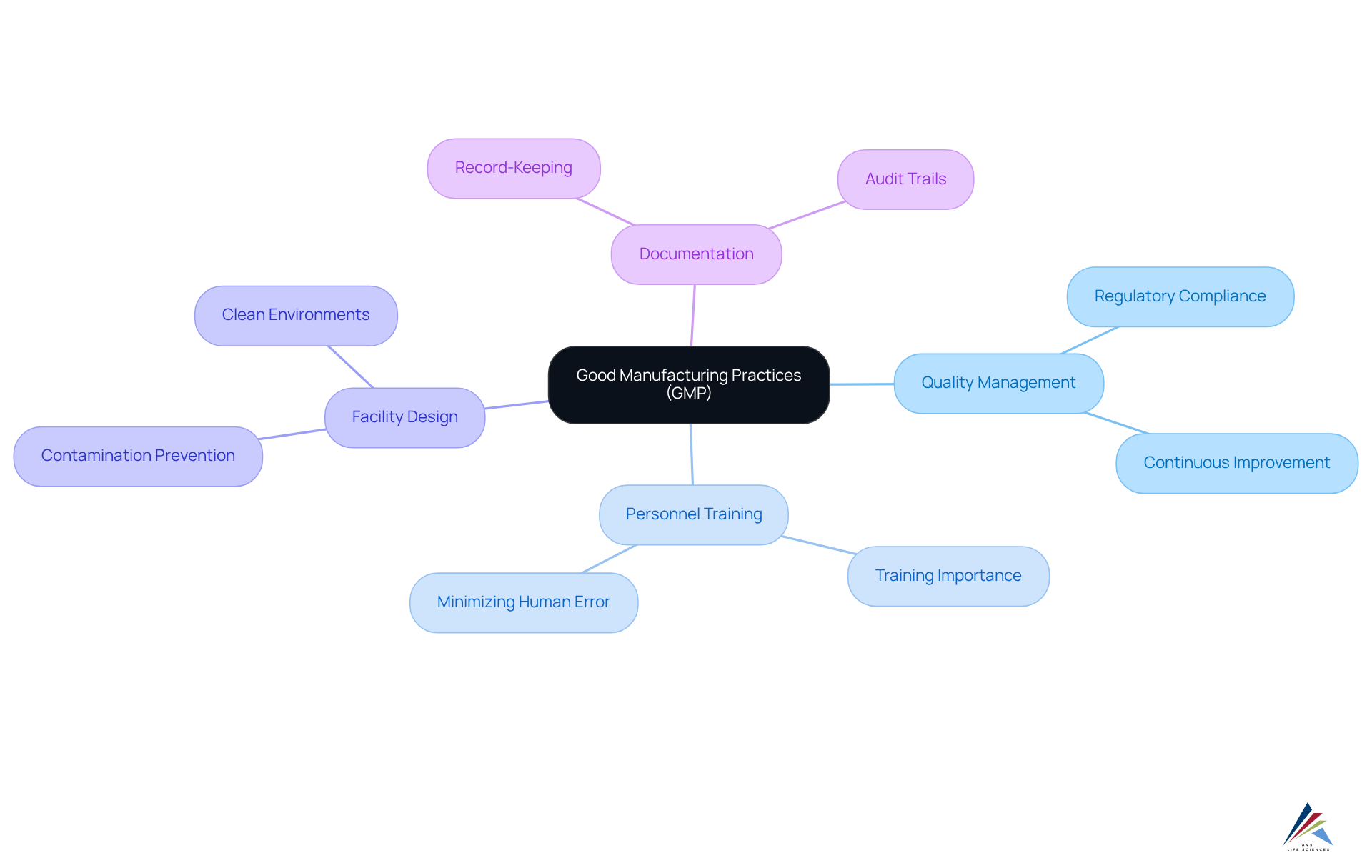
The gmp logo represents essential Good Manufacturing Practices (GMP) that serve as guidelines to ensure products are consistently produced and controlled according to stringent quality standards. These practices hold particular significance in the pharmaceutical and biotechnology sectors, where product safety and efficacy are non-negotiable. Understanding GMP necessitates a grasp of its core principles, which include:
- Quality Management: Establishing a comprehensive quality management system that oversees all production aspects is crucial. This system guarantees adherence to regulatory requirements, including GXP and FDA regulations, while promoting a culture of continuous improvement.
- Personnel Training: Adequate training for all employees in GMP protocols is fundamental. A skilled workforce significantly contributes to maintaining high-quality standards and minimizing human error, which is critical in complex manufacturing processes. Ongoing education and flexibility are essential to overcoming GMP regulatory challenges.
- Facility Design: Facilities must be designed to minimize contamination risks and ensure product integrity. This involves maintaining clean and organized environments, which are vital for preventing cross-contamination and ensuring adherence to the standards represented by the gmp logo. The five P's of GMP—Premises, People, Products, Processes, and Procedures—are central to the guidelines represented by the GMP logo.
- Documentation: Thorough documentation is mandatory to trace every step of the manufacturing process. A systematic record-keeping system not only offers evidence of adherence but also creates a historical archive essential during audits and inspections. Regular inspections are necessary to maintain quality control standards and ensure adherence to GMP.
Adhering to the GMP logo can significantly reduce the risk of product recalls, which disrupt supply chains and affect the availability of critical medications. Companies that implement robust quality management systems and conduct regular inspections have demonstrated improved operational efficiency and reduced recall rates. Furthermore, recent updates to GMP guidelines emphasize the need for continuous adaptation to evolving regulations, ensuring that companies remain competitive and compliant in a rapidly changing industry. Ultimately, a strong commitment to the GMP logo not only safeguards consumer health but also boosts a company's reputation and operational success. Non-compliance with GMP can lead to severe consequences, including fines and production shutdowns, underscoring the importance of these practices.
Identify Regulatory Standards for GMP Logo Design
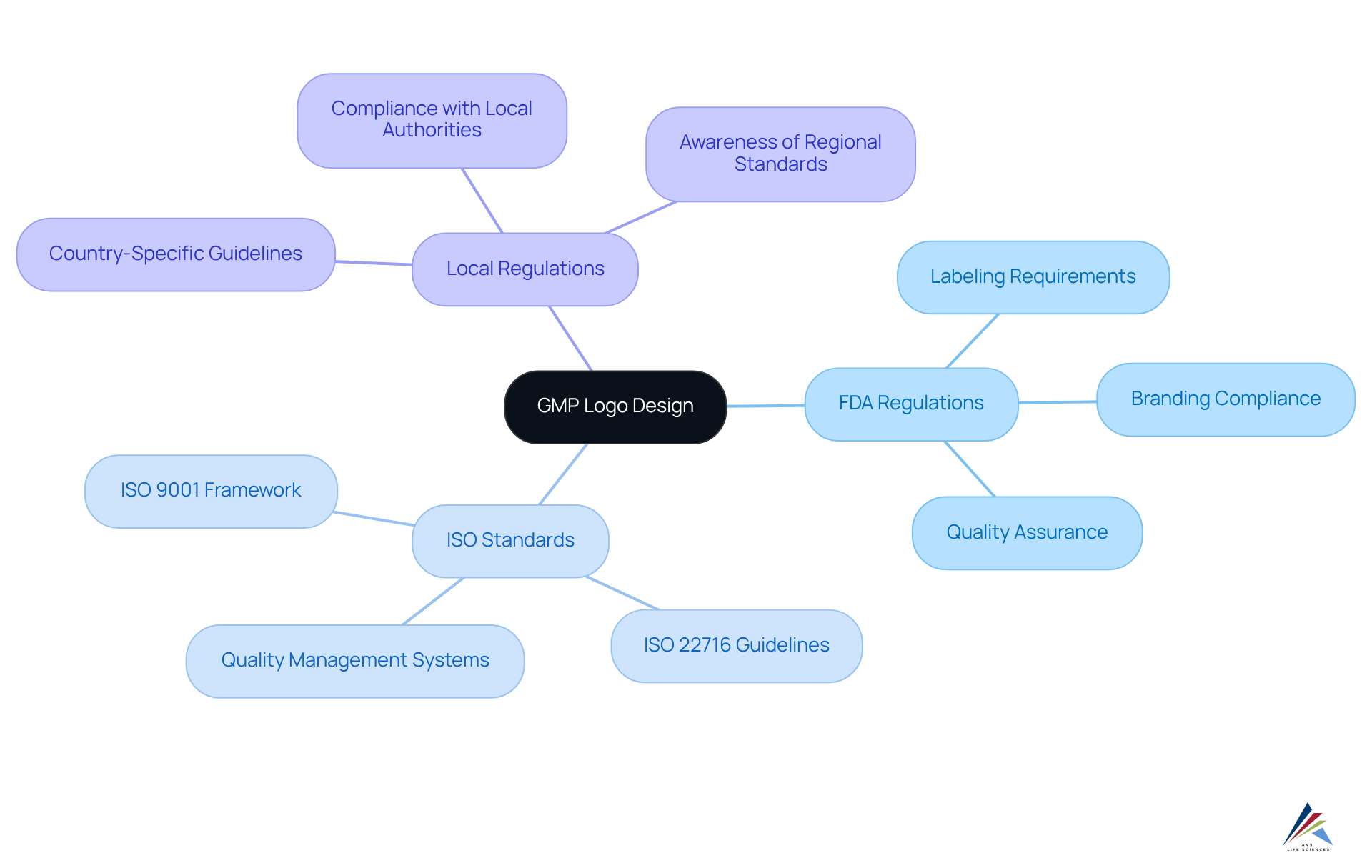
Creating a GMP emblem necessitates a comprehensive understanding of the relevant regulatory standards, which can differ by region. Key considerations include:
- FDA Regulations: The Food and Drug Administration (FDA) in the United States delineates detailed requirements for labeling and branding within the pharmaceutical sector, ensuring that logos effectively convey compliance and quality.
- [ISO Standards](https://avslifesciences.com/industry-news/cders-quality-management-maturity-program): Adhering to ISO standards, particularly ISO 9001, is essential as it establishes a framework for quality management systems that can significantly enhance the credibility of the gmp logo.
- Local Regulations: Various nations may impose additional requirements through local authorities, necessitating awareness of these specific guidelines.
To achieve compliance, designers should routinely consult the latest directives from these . Engaging with regulatory specialists for design evaluations is also advisable; this proactive strategy greatly reduces the risk of non-compliance and bolsters the brand's credibility in the marketplace.
Design the GMP Logo: Key Considerations and Best Practices
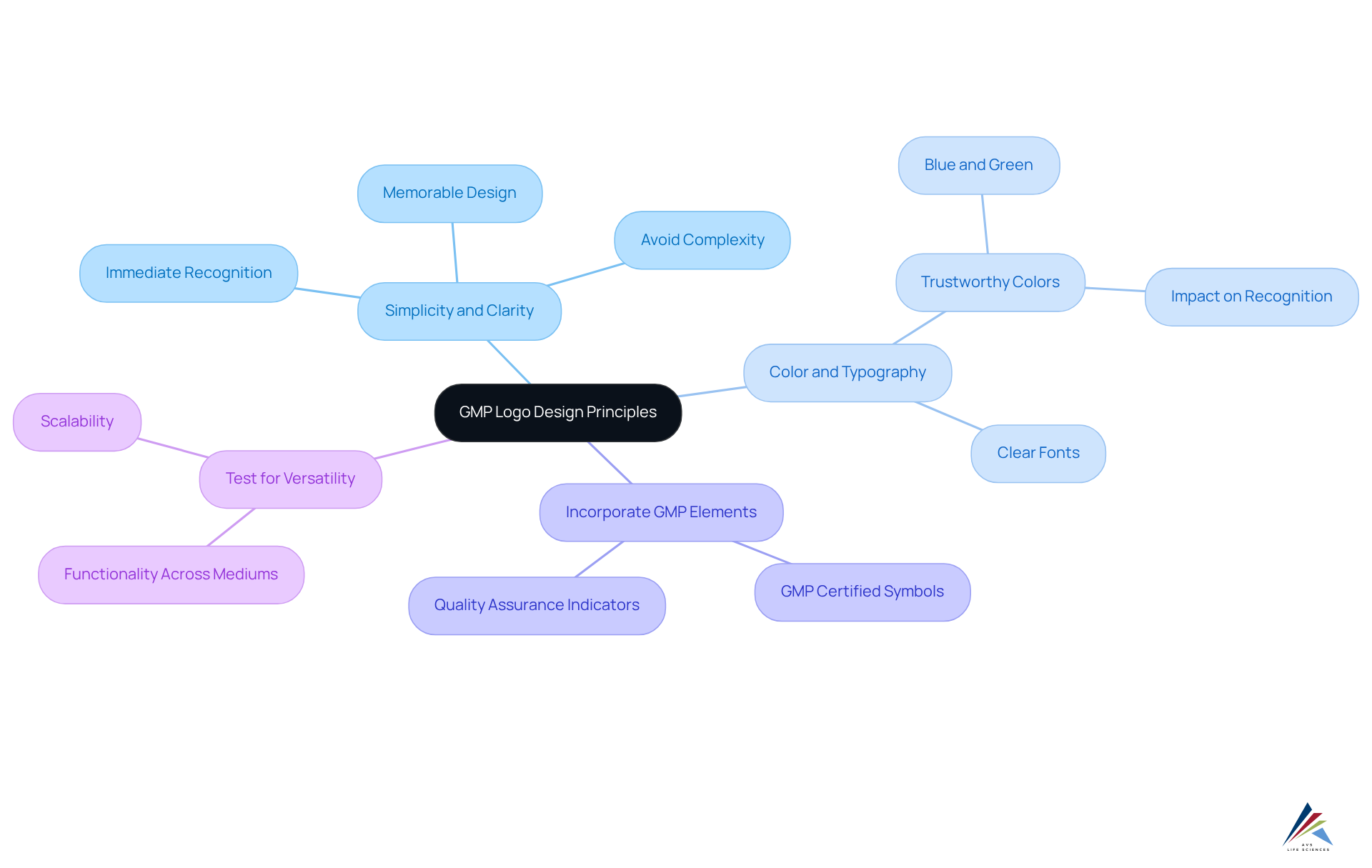
Designing a GMP logo demands meticulous attention to several key principles that not only enhance compliance but also bolster recognition of the GMP logo among consumers.
- Simplicity and Clarity: A successful GMP logo must embody simplicity and clarity, facilitating immediate recognition and understanding. Overly complex designs can obscure the brand's message and confuse consumers; thus, prioritizing straightforward visuals is essential.
- Color and Typography: The selection of colors that evoke trust and professionalism is crucial. Shades like blue and green are often associated with reliability within the pharmaceutical industry. Research indicates that color can improve brand recognition by 80%, underscoring the importance of careful color choices in design. Moreover, utilizing clear fonts ensures that the design remains comprehensible across varying sizes and formats.
- Incorporate GMP Elements: Including elements that signify compliance, such as 'GMP Certified' or symbols representing quality assurance, reinforces the brand's commitment to standards. This approach not only enhances credibility but also effectively communicates the brand's .
- Test for Versatility: A well-crafted emblem must function effectively across various mediums, including digital and print. It should be scalable, maintaining clarity whether displayed on a small label or a large billboard.
By adhering to these design principles, companies can create a GMP logo that not only meets regulatory requirements but also connects with consumers. Brands that employ consistent color palettes can experience a 33% increase in recognition, particularly in the competitive pharmaceutical landscape. Successful examples, such as The Ordinary and Muji, illustrate how simplicity in design can effectively convey brand values and enhance consumer trust. Investing in a professional symbol is imperative for businesses, as it plays a critical role in establishing brand identity and recognition.
Validate and Approve the GMP Logo for Compliance
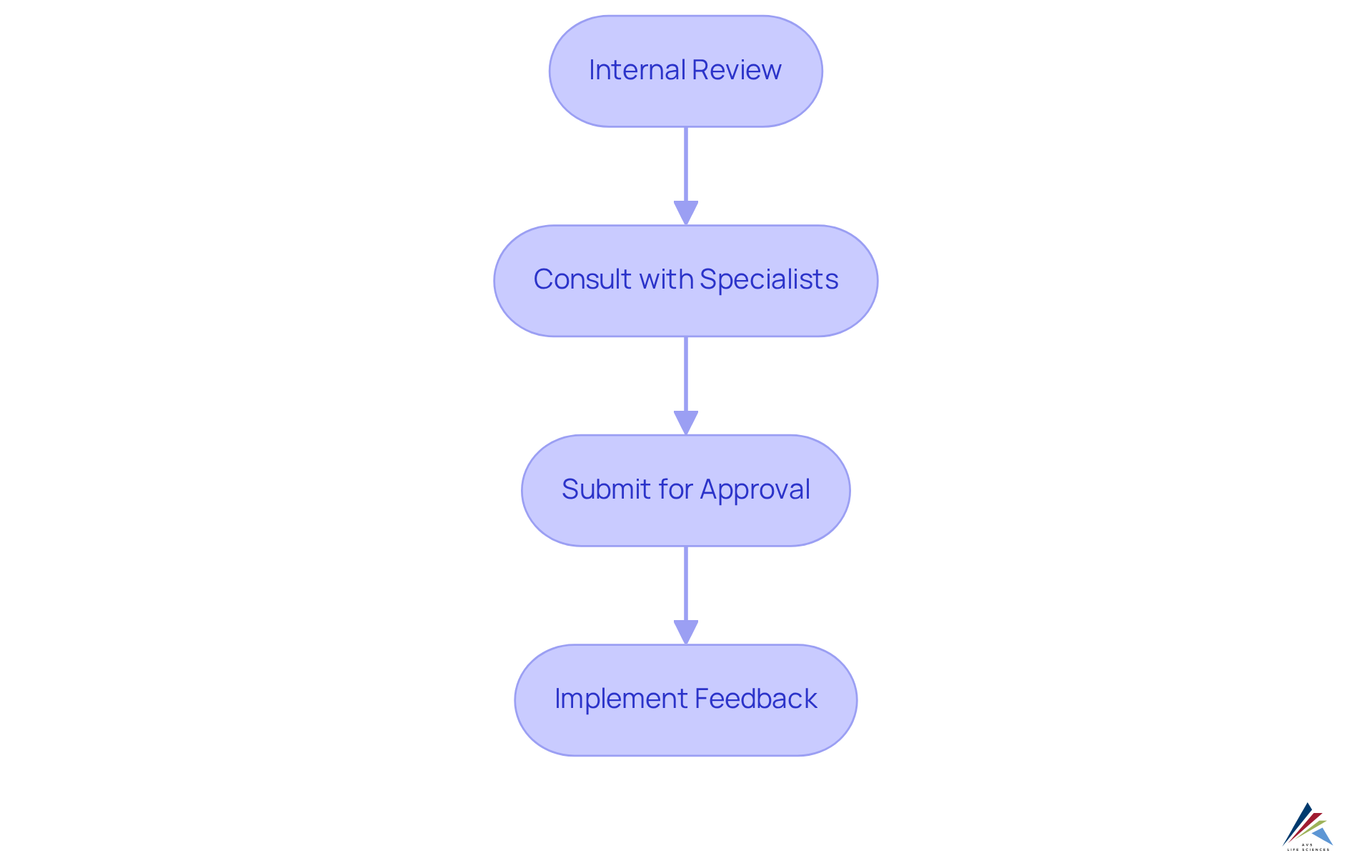
Confirming and endorsing the GMP symbol for conformity is a crucial procedure that guarantees alignment with established standards. This process typically involves several key steps:
- Internal Review: Initiate an internal review of the logo to ensure it aligns with regulatory standards and company policies. This step should involve collecting input from key stakeholders, including regulatory and marketing teams, to ensure a thorough assessment.
- Consult with Specialists: Involve professionals in oversight to examine the design. Their expertise can help identify potential regulatory issues, such as documentation errors or misalignment with GMP principles, before submission.
- Submit for Approval: If necessary, present the design to relevant regulatory bodies for approval. This submission may need comprehensive documentation showing how the emblem meets , which is essential for preventing common regulatory pitfalls.
- Implement Feedback: Be prepared to adjust the design based on feedback received during the review process. This repetitive method not only improves adherence but also guarantees that the final emblem effectively conveys the firm’s dedication to quality.
By meticulously following these validation steps, companies can confidently launch their gmp logo, ensuring that it meets industry standards and regulatory requirements while reinforcing their dedication to quality and compliance.
Conclusion
Mastering the design of a GMP logo is crucial for companies seeking to convey their unwavering commitment to quality and compliance within the manufacturing sector. By grasping the essence of Good Manufacturing Practices and their significance, businesses can craft logos that not only adhere to regulatory requirements but also resonate deeply with consumers. The foundational principles of quality management, personnel training, facility design, and meticulous documentation underscore the importance of the GMP logo, bolstering a brand's dedication to safety and efficacy.
Key arguments presented highlight the necessity of conforming to regulatory standards, such as FDA regulations and ISO guidelines, which shape the design process. The significance of simplicity, clarity, appropriate color choices, and the integration of GMP elements into the logo cannot be overstated, as these factors substantially enhance brand recognition and consumer trust. Additionally, the validation and approval process guarantees that the logo aligns with established standards, safeguarding against potential compliance issues.
In conclusion, dedicating time and resources to mastering GMP logo design transcends mere compliance; it is about forging a robust brand identity that prioritizes consumer safety and quality. As the regulatory landscape evolves, companies must remain vigilant and adaptable, ensuring that their logos authentically reflect their commitment to excellence. Embracing these best practices will not only elevate brand reputation but also contribute significantly to the overall success and reliability of products in the marketplace.
Frequently Asked Questions
What are Good Manufacturing Practices (GMP)?
Good Manufacturing Practices (GMP) are guidelines that ensure products are consistently produced and controlled according to stringent quality standards, particularly in the pharmaceutical and biotechnology sectors.
Why are GMP important in the pharmaceutical and biotechnology industries?
GMP are crucial in these industries because they ensure product safety and efficacy, which are non-negotiable for consumer health.
What are the core principles of GMP?
The core principles of GMP include Quality Management, Personnel Training, Facility Design, and Documentation.
How does Quality Management contribute to GMP?
Quality Management involves establishing a comprehensive system that oversees all production aspects, ensuring adherence to regulatory requirements and promoting a culture of continuous improvement.
Why is Personnel Training important in GMP?
Adequate training for employees in GMP protocols is fundamental as it minimizes human error and maintains high-quality standards in complex manufacturing processes.
What role does Facility Design play in GMP?
Facility Design is essential for minimizing contamination risks and ensuring product integrity, which involves maintaining clean and organized environments.
What are the five P's of GMP?
The five P's of GMP are Premises, People, Products, Processes, and Procedures, which are central to the guidelines represented by the GMP logo.
Why is documentation necessary in GMP?
Thorough documentation is mandatory to trace every step of the manufacturing process, providing evidence of adherence and creating a historical archive for audits and inspections.
How can adherence to GMP reduce product recalls?
Adhering to GMP significantly reduces the risk of product recalls, which can disrupt supply chains and affect the availability of critical medications.
What are the consequences of non-compliance with GMP?
Non-compliance with GMP can lead to severe consequences, including fines and production shutdowns, highlighting the importance of these practices for companies.
