Mastering FDA Cosmetic Recall Authority: A Step-by-Step Guide

Overview
The article centers on the FDA's authority concerning cosmetic recalls, particularly highlighting the advancements introduced by the Modernization of Cosmetics Regulation Act (MoCRA). It clarifies that the FDA now possesses the power to mandate recalls for adulterated or misbranded products.
Manufacturers are required to adhere to new regulations, which include:
- Enhanced reporting
- Safety evaluations
These regulations are aimed at safeguarding consumer safety and mitigating legal repercussions. This shift underscores the critical need for compliance in the evolving regulatory landscape.
Introduction
The evolving landscape of cosmetic regulation is increasingly shaped by the FDA's authority, particularly in the wake of the Modernization of Cosmetics Regulation Act (MoCRA). As manufacturers confront the complexities of compliance, grasping the nuances of FDA recall authority becomes paramount for safeguarding consumer safety and preserving brand integrity.
How can companies adeptly navigate these stringent regulations while effectively managing the risks associated with potential product withdrawals?
This article explores the critical steps and strategies that producers must adopt to master the intricacies of FDA cosmetic recalls, ensuring they are well-prepared for any challenges that may arise.
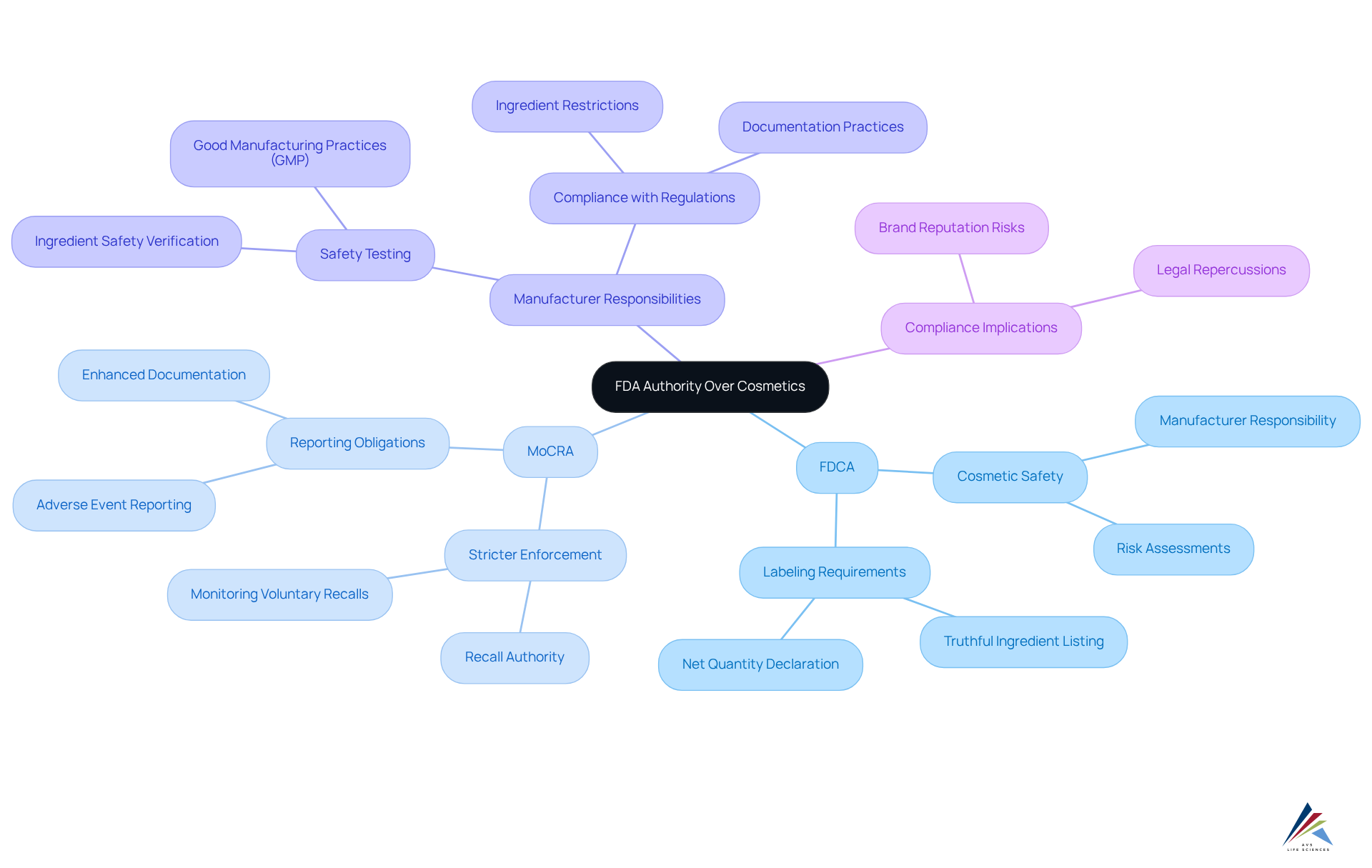
Clarify FDA Authority Over Cosmetics
The FDA's authority over cosmetics is primarily established by the Federal Food, Drug, and Cosmetic Act (FDCA). Unlike pharmaceuticals, cosmetics from the FDA; however, manufacturers are legally responsible for ensuring their items are safe for consumer use and accurately labeled. The FDA cosmetic recall authority allows the agency to act against cosmetics that are considered adulterated or misbranded, which can involve seizing products or initiating withdrawals.
The Modernization of Cosmetics Regulation Act of 2022 (MoCRA) significantly enhances the FDA cosmetic recall authority, enabling stricter enforcement of regulations and the power to mandate recalls when necessary. This legislative shift underscores the importance of compliance for manufacturers, as failure to adhere to these regulations can lead to legal repercussions and damage to brand reputation.
Key provisions of the FDCA mandate that all cosmetic items be safe and that their labels offer honest and non-deceptive information. The MoCRA introduces new requirements, including enhanced reporting obligations and stricter Good Manufacturing Practices (GMP), which are anticipated to improve overall safety and consumer trust.
Case studies demonstrate the effect of these regulations: producers must now perform comprehensive risk evaluations and maintain meticulous records of formulations and safety testing. As the regulatory landscape evolves, companies must adapt proactively to ensure compliance and safeguard their market position. AVS Life Sciences provides extensive quality management and regulatory compliance solutions that can aid manufacturers in navigating these complex regulations, ensuring that their offerings meet safety standards and uphold consumer trust.
Examine FDA Recall Authority for Cosmetics
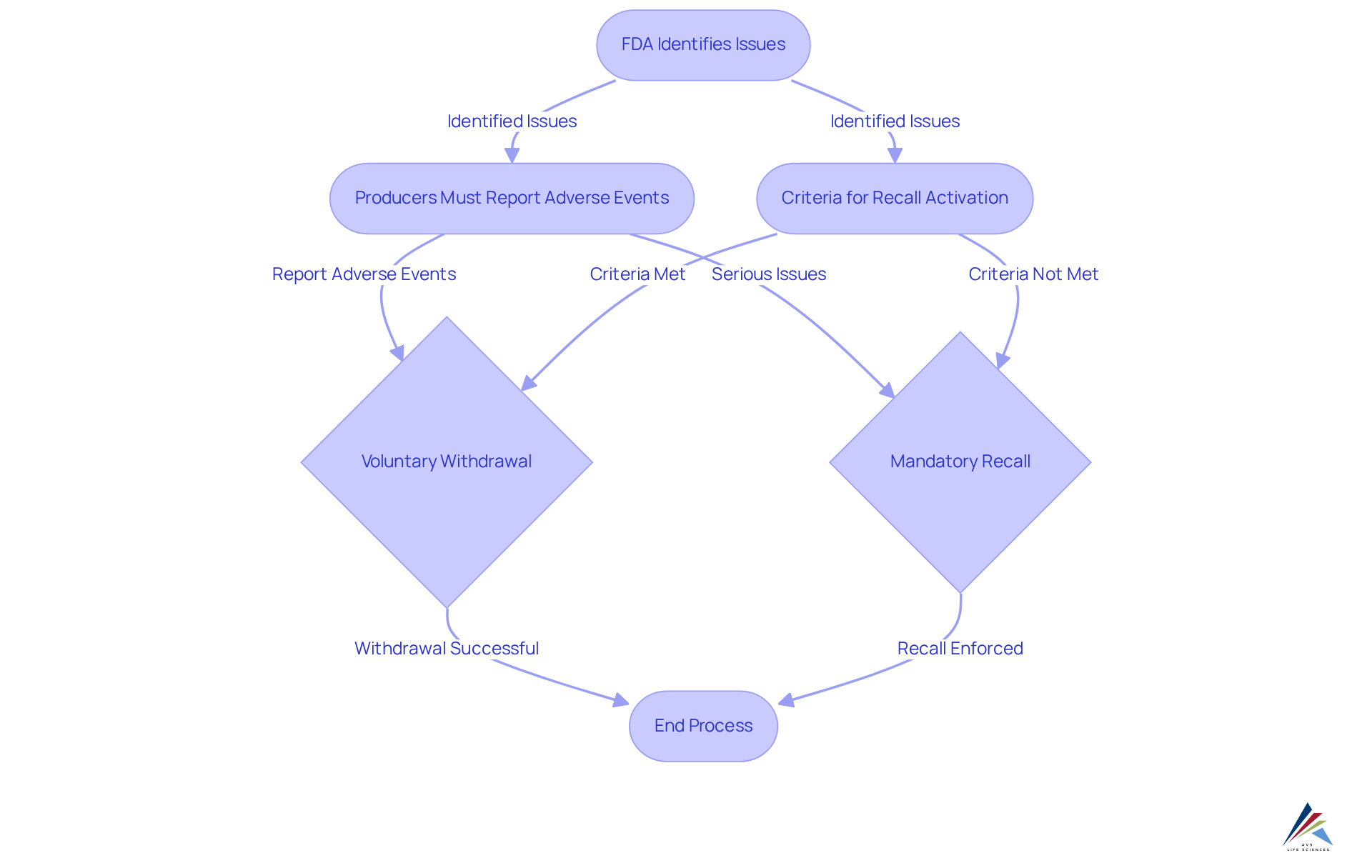
Under the Modernization of Cosmetics Regulation Act (MoCRA), the FDA cosmetic recall authority grants the agency the power to compel the withdrawal of cosmetics. This pivotal authority, known as the FDA cosmetic recall authority, allows the FDA to order a recall if it identifies a reasonable probability that a cosmetic product is adulterated or misbranded. Such a shift from voluntary adherence to obligatory measures compels producers to act decisively to protect consumers. Companies must remain vigilant regarding the criteria that activate this authority, particularly concerning safety and labeling issues, to ensure they can respond effectively.
Producers are mandated to report serious adverse events to the FDA within 15 business days, underscoring the urgency of compliance. Furthermore, the FDA can request ingredient lists for fragrances or flavors associated with serious adverse events, emphasizing the critical need for safety substantiation. Should a voluntary withdrawal be declined, the FDA cosmetic recall authority allows for the enforcement of a mandatory removal, highlighting the severe repercussions of non-compliance.
Experts in the field recognize that the implications of MoCRA transcend immediate compliance; they necessitate that producers implement proactive strategies for safety substantiation and risk management. As the FDA amplifies its oversight capabilities, companies must prioritize transparency and meticulous documentation to adeptly navigate the complexities of regulatory compliance. Understanding these dynamics is crucial for producers aiming to and ensure consumer safety.
Outline Responsibilities During a Cosmetic Recall
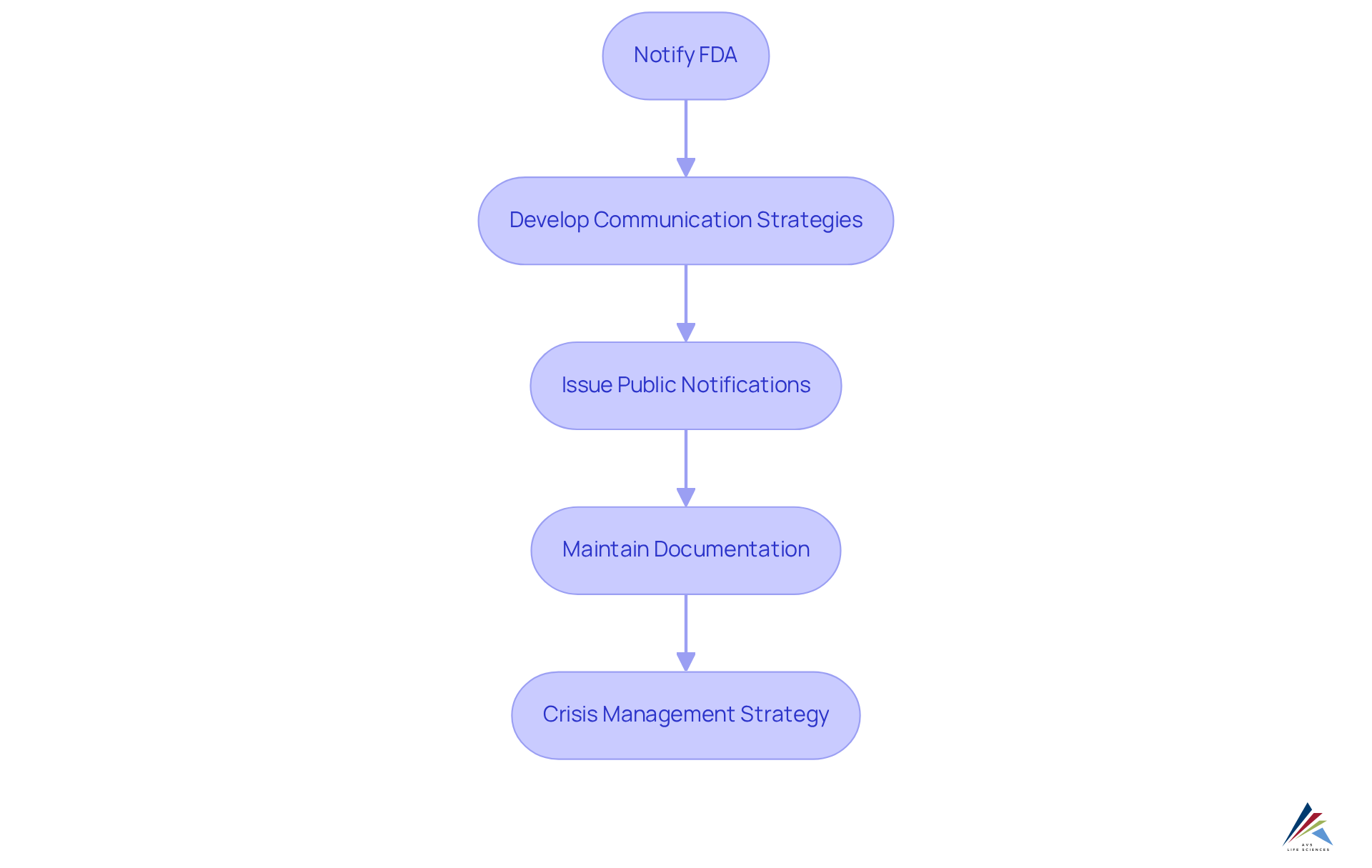
During a cosmetic product withdrawal, producers face essential duties critical for ensuring compliance with the FDA cosmetic recall authority and safeguarding consumer safety. Timely communication with the FDA cosmetic recall authority is paramount, requiring comprehensive details about the product, the reasons for withdrawal, and any associated hazards. While the duration for can vary, prompt action is vital to mitigate potential health risks. Furthermore, companies must develop effective communication strategies with retailers and consumers, ensuring that recalled items are promptly removed from shelves and that consumers are adequately informed about the withdrawal. This involves issuing public notifications that comply with FDA requirements, which may necessitate submitting a statement and distribution strategy for the FDA cosmetic recall authority review.
Documentation is integral to the retrieval process; maintaining precise records not only aids in compliance but also serves as a safeguard against potential legal scrutiny. Additionally, businesses should devise a crisis management strategy to handle public relations effectively during a product withdrawal, ensuring that messaging remains clear and consistent. Expert insights suggest that a well-defined retrieval strategy, which includes assigning lot numbers for easier identification and conducting effectiveness checks per regulatory guidelines, can significantly enhance the retrieval process. By prioritizing these responsibilities, producers can navigate the complexities of cosmetic withdrawals while protecting public health. Moreover, leveraging AVS Life Sciences' expertise in quality management and regulatory compliance can provide manufacturers with tailored solutions to streamline their product retrieval processes and ensure adherence to all regulatory requirements.
Implement Effective Recall Strategies and Procedures
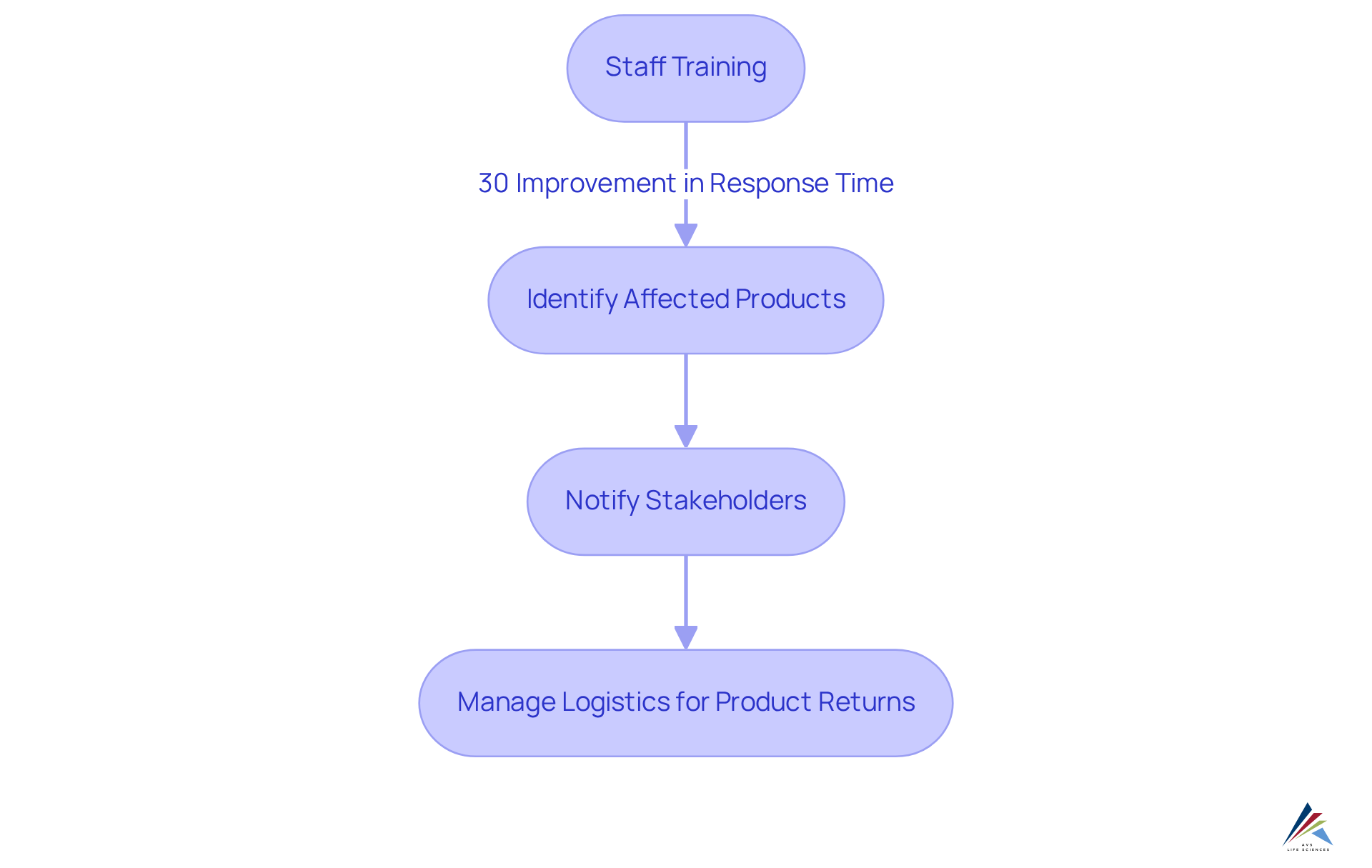
To execute effective retrieval strategies, companies must develop a comprehensive recovery plan that encompasses several essential elements. This plan should delineate clear procedures for:
- Identifying affected products
- Notifying stakeholders
- Managing logistics for product returns
Training staff on retrieval procedures is paramount; research indicates that can enhance response times by as much as 30%, ensuring that all team members fully understand their responsibilities during a retrieval. Industry leaders underscore that well-trained staff are vital for minimizing confusion and maintaining operational efficiency in such crises. Recent studies reveal that providing a complete solution can boost memory effectiveness by 11.4% compared to partial solutions, underscoring the importance of thorough strategies.
A robust communication strategy is equally critical, involving timely updates to consumers and the media to foster transparency and trust. Regularly assessing and evaluating the retrieval plan through simulations can help identify potential vulnerabilities, with organizations that engage in these exercises reporting a 25% improvement in response times during real incidents. Examples of comprehensive withdrawal strategies in the cosmetic sector, as outlined by the FDA cosmetic recall authority, include those implemented by major brands, which typically feature detailed procedures for:
- Consumer communication
- Product recovery
- Post-withdrawal evaluation to prevent future occurrences
By prioritizing these essential components, companies can significantly enhance their recall effectiveness and protect their reputation.
Conclusion
The FDA's authority over cosmetic recalls is a critical aspect of ensuring consumer safety and maintaining industry integrity. With the enactment of the Modernization of Cosmetics Regulation Act (MoCRA), the agency's ability to enforce compliance has significantly strengthened, compelling manufacturers to adhere to stringent safety and labeling standards. This shift emphasizes the necessity for companies to take proactive measures in managing their products and understanding their legal obligations.
Key responsibilities of producers during a recall include:
- Timely communication with the FDA
- Meticulous documentation
- Implementation of effective retrieval strategies
The importance of training staff and developing comprehensive recovery plans cannot be overstated; these elements are vital for minimizing confusion and ensuring operational efficiency during a crisis. Moreover, industry leaders can leverage expert insights and resources to navigate the complexities of regulatory compliance and enhance their recall processes.
Ultimately, mastering the FDA's cosmetic recall authority transcends mere compliance; it fosters a culture of safety and transparency that prioritizes consumer trust. Companies are encouraged to embrace these regulations as an opportunity to strengthen their brand reputation and ensure the well-being of their customers. By adopting best practices and remaining vigilant in their compliance efforts, manufacturers can mitigate risks associated with recalls and contribute to a safer and more reliable cosmetic industry.
Frequently Asked Questions
What establishes the FDA's authority over cosmetics?
The FDA's authority over cosmetics is primarily established by the Federal Food, Drug, and Cosmetic Act (FDCA).
Do cosmetics require pre-market approval from the FDA?
No, unlike pharmaceuticals, cosmetics do not require pre-market approval from the FDA.
What responsibilities do manufacturers have regarding cosmetics?
Manufacturers are legally responsible for ensuring their cosmetics are safe for consumer use and accurately labeled.
What actions can the FDA take against adulterated or misbranded cosmetics?
The FDA can seize products or initiate withdrawals of cosmetics that are considered adulterated or misbranded.
What is the Modernization of Cosmetics Regulation Act of 2022 (MoCRA)?
The MoCRA significantly enhances the FDA's cosmetic recall authority, enabling stricter enforcement of regulations and the power to mandate recalls when necessary.
What are the implications of failing to comply with cosmetic regulations?
Failure to adhere to regulations can lead to legal repercussions and damage to a brand's reputation.
What key provisions does the FDCA mandate for cosmetics?
The FDCA mandates that all cosmetic items be safe and that their labels provide honest and non-deceptive information.
What new requirements does MoCRA introduce for cosmetic manufacturers?
MoCRA introduces enhanced reporting obligations and stricter Good Manufacturing Practices (GMP).
How do these regulations affect the safety and consumer trust in cosmetics?
The new requirements are anticipated to improve overall safety and enhance consumer trust in cosmetic products.
What do case studies indicate about the impact of these regulations on producers?
Producers must now perform comprehensive risk evaluations and maintain meticulous records of formulations and safety testing.
How can companies ensure compliance with evolving regulations?
Companies must adapt proactively to ensure compliance and safeguard their market position.
What services does AVS Life Sciences provide to manufacturers?
AVS Life Sciences offers extensive quality management and regulatory compliance solutions to help manufacturers navigate complex regulations and meet safety standards.
