Master Validation Lifecycle Management for Compliance Success

Overview
Mastering validation lifecycle management is crucial for achieving compliance success in life sciences. This process encompasses critical phases such as:
- Planning
- Qualification
- Verification
All of which ensure that products meet stringent regulatory standards. A comprehensive validation master plan, coupled with rigorous qualification protocols, significantly enhances operational efficiency, reduces verification times, and bolsters readiness for audits. This, in turn, safeguards public health and ensures product quality. The importance of these elements cannot be overstated, as they form the backbone of a robust compliance strategy that not only meets but exceeds industry expectations.
Introduction
Mastering validation lifecycle management transcends mere regulatory compliance; it stands as a fundamental pillar of success within the life sciences industry. By adeptly navigating the intricate phases of planning, qualification, and verification, organizations can ensure their systems consistently deliver high-quality products while adhering to compliance standards.
Nonetheless, numerous entities struggle with the dual challenge of maintaining efficiency and accuracy in their validation processes. What strategies can organizations implement to transform their validation lifecycle into a streamlined, compliant, and perpetually improving system?
Understand the Validation Lifecycle in Life Sciences
The validation lifecycle management in life sciences encompasses several critical phases: planning, qualification, and verification. Each phase plays a pivotal role in ensuring that systems and equipment consistently produce products that meet established specifications.
- Planning: This foundational phase involves defining the assessment scope, identifying essential procedures, and establishing verification protocols. A well-organized Master Plan (VMP) is crucial, as it outlines the verification strategy, resources, and timelines necessary for successful execution. Organizations that prioritize a comprehensive VMP often experience more seamless approval processes and enhanced compliance outcomes. For instance, a pharmaceutical firm that implemented a thorough VMP reported a 30% reduction in verification time.
- Qualification: This stage includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ confirms that equipment is installed correctly, OQ ensures it operates according to specifications, and PQ verifies its effectiveness under real-world conditions. Firms that adopt rigorous qualification protocols tend to report fewer deviations and enhanced operational efficiency. For example, a biotech company that adhered to strict OQ protocols observed a significant decline in equipment failures during production runs.
- Verification: The final phase involves and meticulously documenting the results. This documentation is vital for regulatory compliance and serves as evidence that procedures can consistently yield quality products. Organizations that maintain comprehensive verification records are better prepared for audits and regulatory inspections. Utilizing a thorough checklist for Computer System Verification (CSV) can further refine the verification process, ensuring that all essential steps are followed and documented.
By understanding and effectively managing these phases through validation lifecycle management, entities can uphold Good Manufacturing Practices (GMP) and other regulatory obligations, ultimately safeguarding public health and ensuring product quality.
Implement Best Practices for Compliance and Efficiency
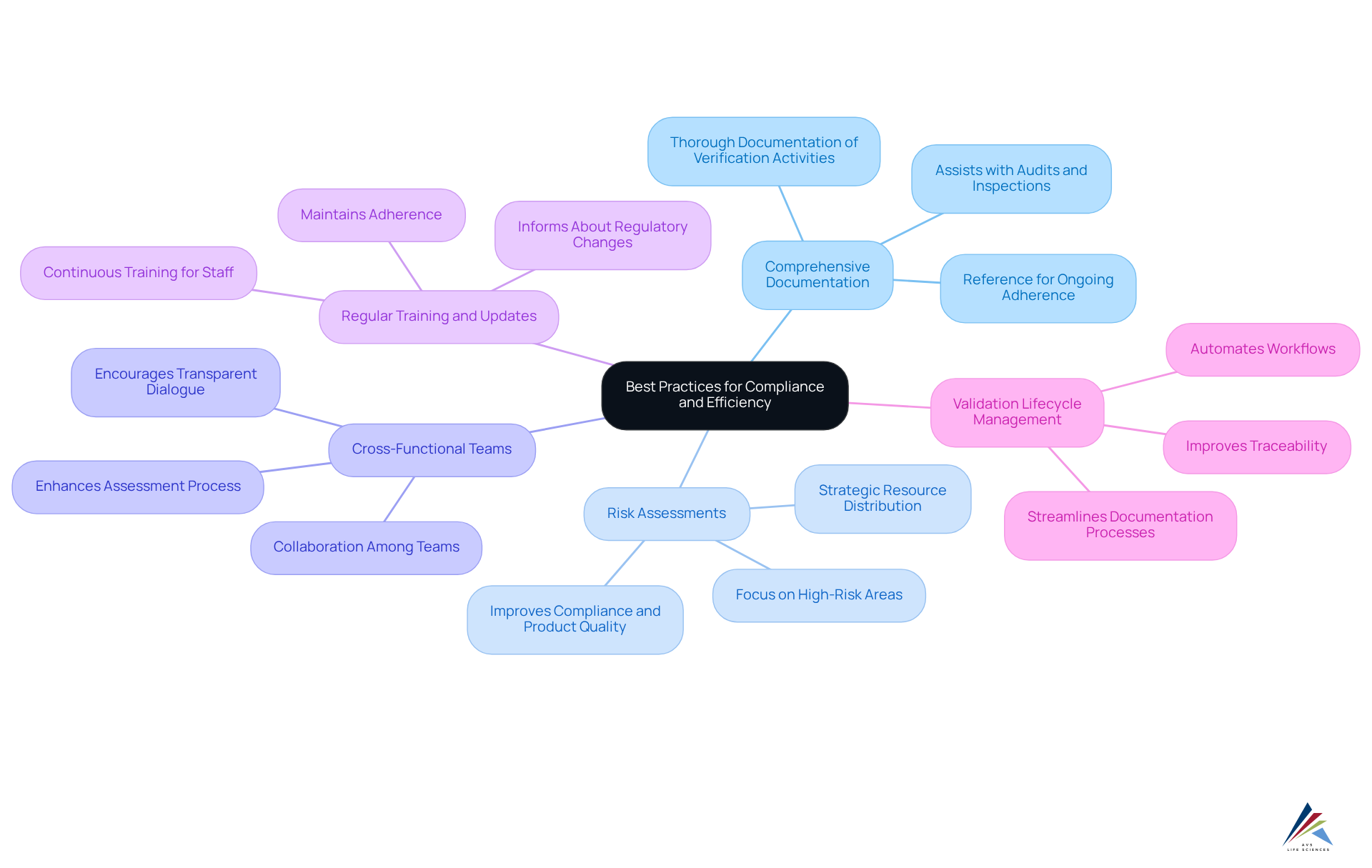
To achieve compliance and efficiency in validation processes, organizations must adopt best practices that address the inherent challenges in this domain:
- Develop Comprehensive Documentation: Thorough documentation of all verification activities—including protocols, results, and deviations—is essential. This documentation not only assists with audits and regulatory inspections but also serves as a crucial reference for ongoing adherence efforts.
- Conduct [Risk Assessments](https://azquotes.com/quotes/topics/compliance.html): By adopting a risk-oriented strategy, entities can focus their assessment efforts on high-risk areas. This strategic distribution of resources ensures that essential activities receive the necessary attention, ultimately improving overall compliance and product quality.
- Engage Cross-Functional Teams: Collaboration among quality assurance, engineering, and production teams is vital. Encouraging transparent dialogue and incorporating varied viewpoints enhances the assessment process and enables entities to tackle potential difficulties more efficiently.
- Regular Training and Updates: Continuous training for staff involved in validation activities is crucial. Keeping the team informed about regulatory changes and best practices ensures that adherence is maintained, allowing the organization to remain agile in adapting to evolving standards.
- Utilize : Implementing can significantly streamline documentation processes, automate workflows, and improve traceability. This technology enables effective management of verification activities, ensuring that compliance requirements are consistently met.
Leverage Automation to Streamline Validation Processes
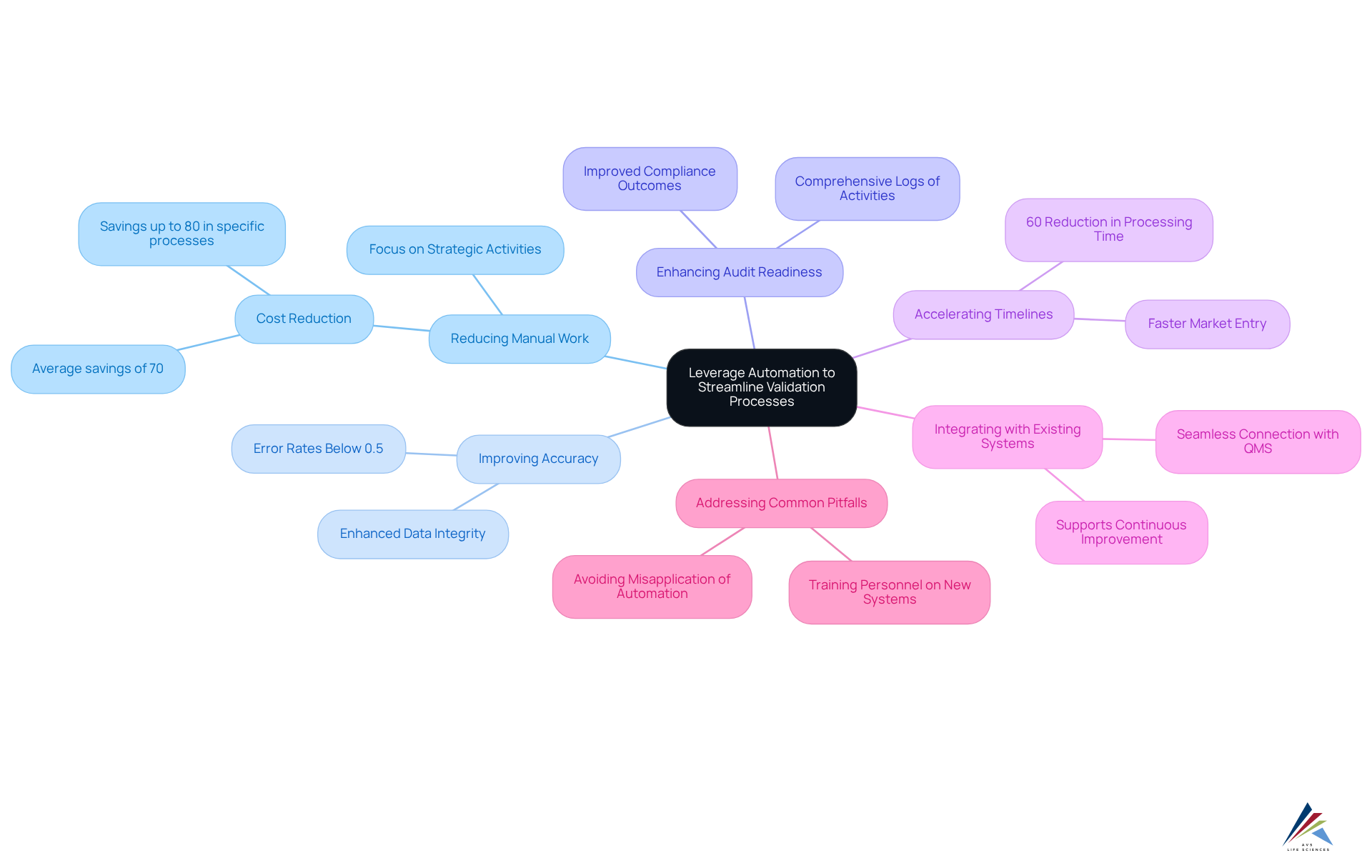
Automation can significantly enhance the validation lifecycle by:
- Reducing Manual Work: Automated systems efficiently manage repetitive tasks like data entry and report generation, allowing validation teams to concentrate on strategic activities that add greater value. Financial institutions implementing end-to-end automation in their back-office operations report an average cost reduction of 70%, with some organizations achieving savings of up to 80% in specific processes. In the life sciences sector, AVS Life Sciences has demonstrated how automation can simplify operations, particularly in adherence to GXP and FDA regulations, while also improving documentation practices and standard operating procedures (SOPs).
- Improving Accuracy: Automated validation tools drastically reduce human error, ensuring data remains consistently accurate and reliable. This precision is crucial in regulated environments where is paramount. As noted by Sarah Lee, organizations leveraging advanced automation technologies report error rates of less than 0.5% in financial operations, compared to an average error rate of 5-10% in manual processing. This level of accuracy is essential for maintaining data integrity in pharmaceutical manufacturing.
- Enhancing Audit Readiness: Automated systems maintain comprehensive logs of all validation activities, simplifying the preparation for audits and inspections. This level of transparency can lead to improved compliance outcomes and reduced regulatory scrutiny. AVS Life Sciences' commitment to exemplary documentation practices and standard operating procedures (SOPs) ensures that clients are always audit-ready.
- Accelerating Timelines: By streamlining workflows and minimizing the time spent on manual tasks, automation enables organizations to meet tight deadlines and expedite product market entry. For example, Bank of America accomplished a 60% decrease in mortgage application processing time via its automated systems, illustrating the potential for comparable efficiencies in verification procedures. AVS Life Sciences has successfully supported clients in upgrading their GMP facilities, allowing them to focus on developing life-saving medicines more efficiently.
- Integrating with Existing Systems: Many contemporary assessment tools seamlessly connect with existing quality management systems (QMS), offering a comprehensive perspective on compliance efforts and enabling informed decision-making. This integration not only enhances operational efficiency but also supports continuous improvement in validation lifecycle management practices, as demonstrated in AVS Life Sciences' approach to managing quality in a virtual company.
- Addressing Common Pitfalls: While automation provides various advantages, companies must be wary of potential drawbacks, such as misapplying automation to unsuitable processes or neglecting to sufficiently train personnel on new systems. A thoughtful approach to automation can help mitigate these challenges, ensuring that compliance and operational excellence are maintained.
Adopt a Continuous Improvement Approach to Validation
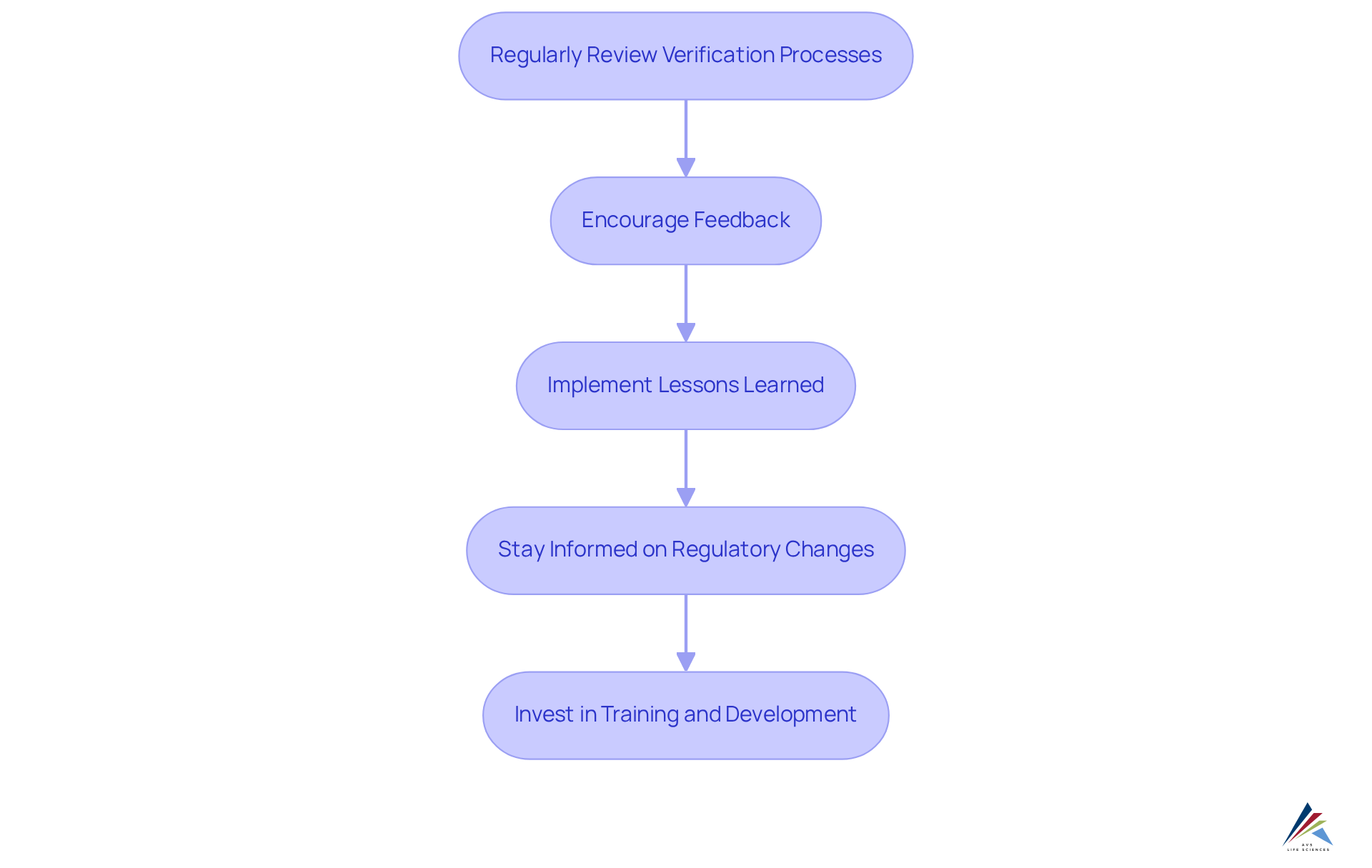
To cultivate a culture of continuous improvement in validation, organizations must take decisive actions:
- Regularly Review Verification Processes: Conduct periodic assessments of verification protocols to identify areas for enhancement. By examining information from earlier assessments, organizations can uncover patterns and shortcomings that require immediate attention.
- Encourage Feedback: Foster an environment where team members feel empowered to provide input on assessment methods. This feedback can yield valuable insights and innovative solutions that drive significant improvement.
- Implement Lessons Learned: Following each assessment project, conduct a thorough review to capture lessons learned. This practice not only assists organizations in avoiding repeated errors but also enhances overall efficiency.
- Stay Informed on Regulatory Changes: Maintain vigilance regarding changes in regulations and industry standards. A proactive approach ensures that verification practices remain compliant and relevant in a dynamic regulatory landscape.
- Invest in Training and Development: Provide ongoing training opportunities for staff to enhance their skills and knowledge. A well-trained team is better equipped to identify improvement opportunities and implement best practices, ultimately leading to more effective validation lifecycle management.
Conclusion
Mastering validation lifecycle management is essential for organizations in the life sciences sector aiming to achieve compliance and operational excellence. Effectively navigating the phases of planning, qualification, and verification allows entities to ensure that their systems and equipment consistently produce high-quality products that meet regulatory standards. A robust validation strategy not only streamlines processes but also significantly reduces the risk of non-compliance, ultimately safeguarding public health.
Key insights from the article highlight the importance of:
- Comprehensive documentation
- Risk assessments
- Cross-functional collaboration
- Continuous training
- Strategic use of automation
Implementing these best practices enables organizations to address challenges in validation processes, enhance efficiency, and maintain readiness for audits and inspections. Moreover, leveraging automation can dramatically reduce manual tasks, improve accuracy, and accelerate timelines, providing a competitive edge in the fast-paced life sciences industry.
As the landscape of life sciences continues to evolve, adopting a continuous improvement approach to validation is paramount. Organizations must remain vigilant in reviewing their processes, encouraging team feedback, and staying informed about regulatory changes. By fostering a culture of ongoing enhancement, entities can not only comply with current standards but also adapt to future challenges, ensuring sustained success in validation lifecycle management. Embracing these strategies will ultimately lead to improved compliance outcomes and a stronger commitment to quality in the life sciences field.
Frequently Asked Questions
What are the main phases of the validation lifecycle in life sciences?
The main phases of the validation lifecycle in life sciences are planning, qualification, and verification.
What is involved in the planning phase of the validation lifecycle?
The planning phase involves defining the assessment scope, identifying essential procedures, and establishing verification protocols. A well-organized Master Plan (VMP) is crucial for outlining the verification strategy, resources, and timelines.
Why is a Master Plan (VMP) important in the validation lifecycle?
A Master Plan (VMP) is important because it helps organizations manage the verification strategy effectively, leading to more seamless approval processes and improved compliance outcomes.
What are the components of the qualification phase?
The qualification phase includes Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). IQ confirms correct installation, OQ ensures operation according to specifications, and PQ verifies effectiveness under real-world conditions.
How does rigorous qualification affect operational efficiency?
Firms that adopt rigorous qualification protocols tend to report fewer deviations and enhanced operational efficiency, leading to better production outcomes.
What does the verification phase entail?
The verification phase involves executing verification protocols and meticulously documenting the results, which is essential for regulatory compliance and quality assurance.
Why is documentation important in the verification phase?
Documentation is vital for regulatory compliance and serves as evidence that procedures can consistently yield quality products, helping organizations prepare for audits and inspections.
How can organizations improve the verification process?
Organizations can improve the verification process by utilizing a thorough checklist for Computer System Verification (CSV), ensuring that all essential steps are followed and documented.
What is the overall benefit of managing the validation lifecycle effectively?
Effectively managing the validation lifecycle helps organizations uphold Good Manufacturing Practices (GMP) and other regulatory obligations, ultimately safeguarding public health and ensuring product quality.
