Master Validation Documentation Digitization for Compliance Success

Overview
This article addresses the critical challenge of mastering the digitization of validation documentation, a key factor in achieving compliance success within regulated industries. Understanding regulatory requirements is paramount; effective digitization strategies must be implemented to navigate these complexities. Furthermore, facilitating training and change management is essential, as these elements collectively enhance compliance and operational efficiency. By leveraging modern document management systems and fostering employee engagement, organizations can significantly improve their compliance posture. Ultimately, the journey towards compliance success is not merely a procedural task but a strategic initiative that demands attention and action.
Introduction
Navigating the intricate landscape of regulatory compliance in the pharmaceutical industry poses a formidable challenge, particularly regarding validation documentation. As organizations endeavor to meet the stringent requirements established by regulatory bodies such as the FDA and ISO, the shift towards digitization emerges not merely as an option but as an imperative for ensuring operational integrity and product quality.
What strategies can companies implement to effectively digitize their validation documentation while surmounting the obstacles of compliance and employee resistance? This article will explore the complexities of compliance, offer detailed solutions, and provide actionable insights that empower organizations to enhance their validation processes.
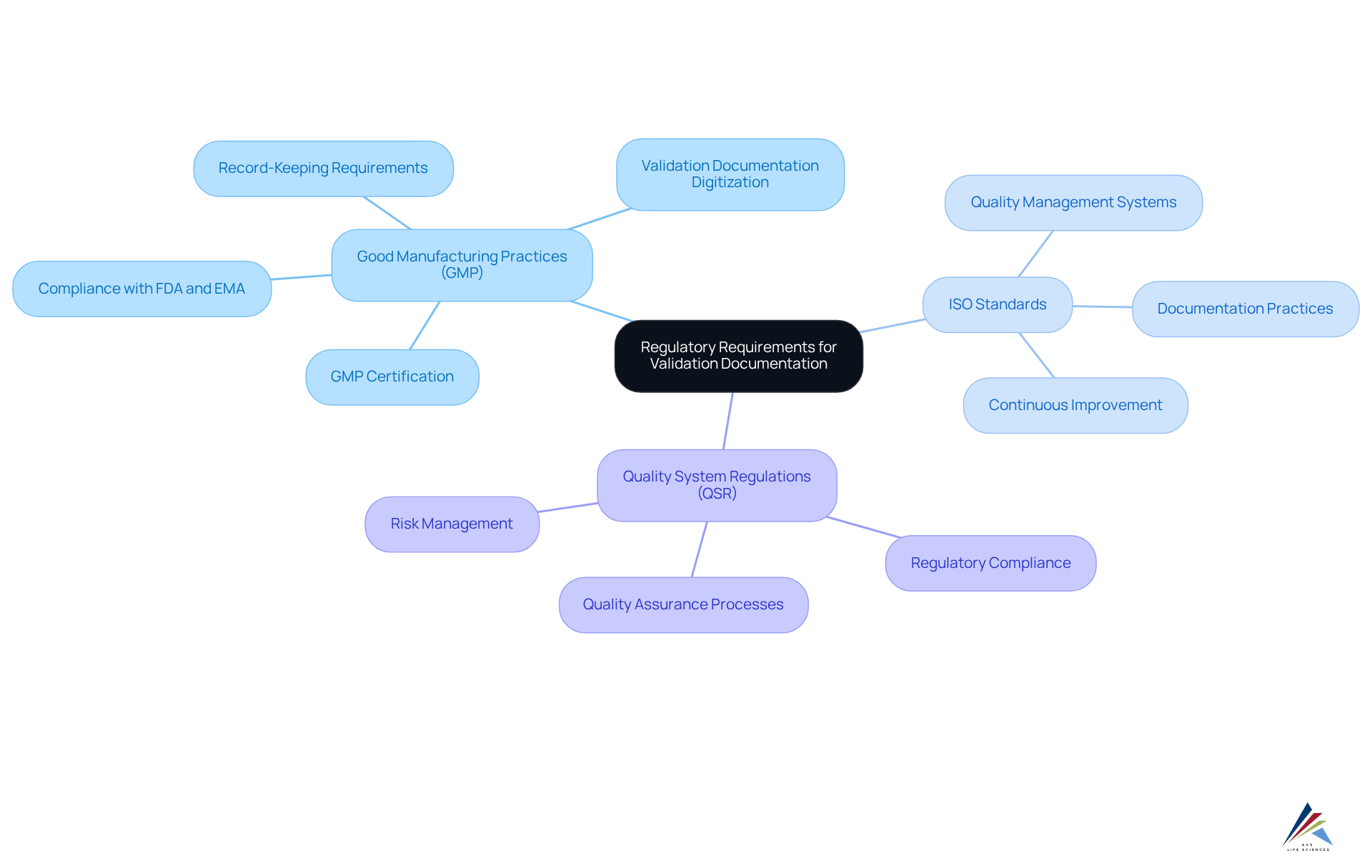
Understand Regulatory Requirements for Validation Documentation
Navigating the complexities of verification records requires a robust understanding of the , particularly the frameworks established by , ISO standards, and . These regulations delineate specific record-keeping requirements that are essential for .
For instance, GMP mandates comprehensive to ensure that that meet established quality standards. Organizations must conduct a meticulous review of relevant regulations and guidelines from authorities such as the FDA and EMA to ensure compliance.
Leveraging resources like the ISPE Good Practice Guide can offer valuable insights tailored to digital verification requirements. By fostering a deep understanding of these regulations, organizations can formulate an effective that aligns with , ultimately enhancing their operational integrity and product quality.
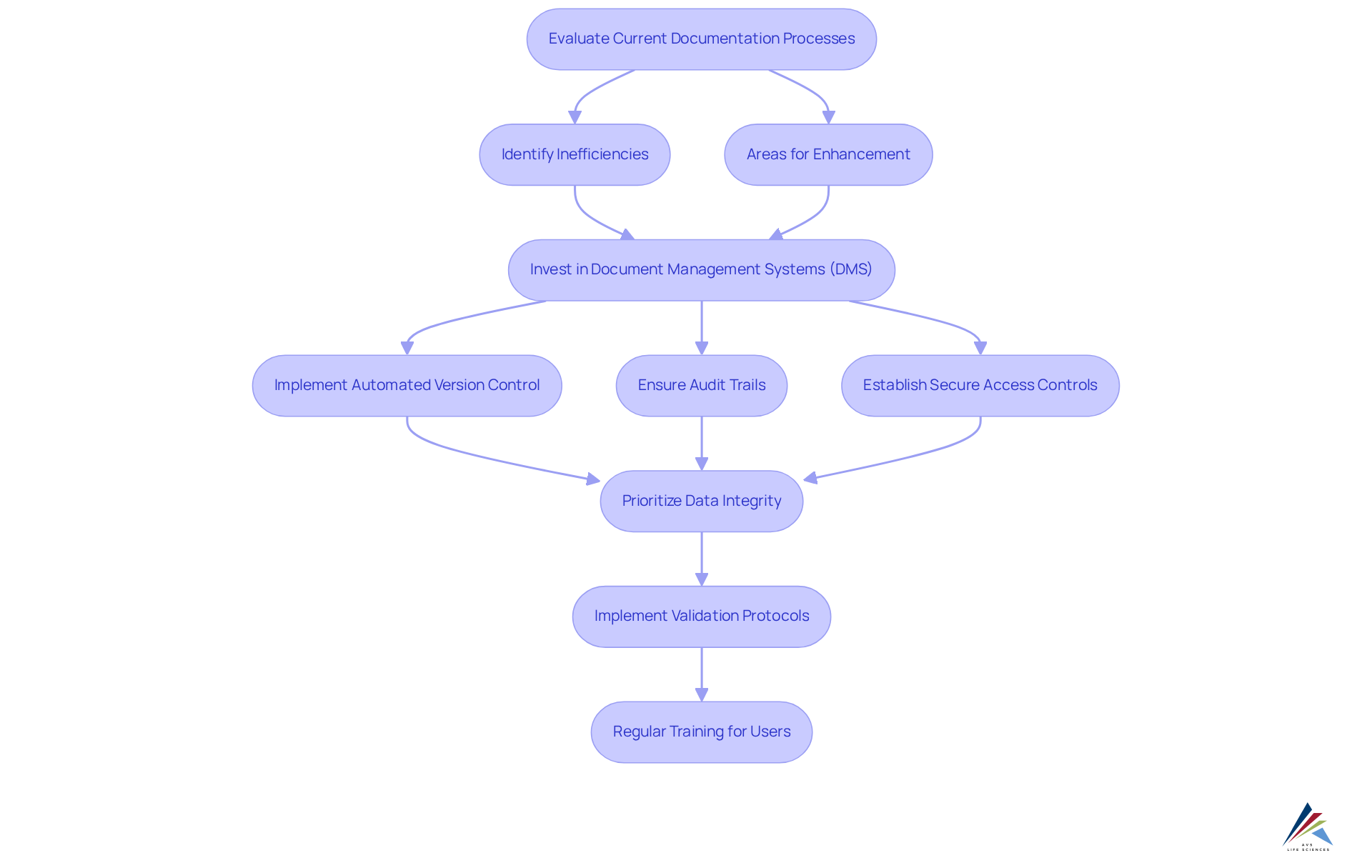
Implement Effective Digitization Strategies for Validation Documentation
To effectively implement , organizations must adopt a structured approach that encompasses the selection of suitable digital tools and the establishment of clear workflows.
Begin by evaluating current documentation processes to pinpoint inefficiencies and areas ripe for enhancement. Investing in (DMS) that include automated version control, audit trails, and secure access controls is essential for the validation documentation digitization required to comply with GXP and FDA regulations. These systems not only strengthen compliance but also foster collaboration among team members.
For instance, cloud-based solutions enable multiple users to access and edit documents concurrently, significantly minimizing the risk of errors. Furthermore, prioritizing ; implementing ensures that all electronic records remain accurate and reliable, in line with best practices for SOPs and CAPA. Regular can enhance user proficiency and ensure adherence to regulatory requirements.
With 79% of companies acknowledging the increasing significance of , the shift to validation documentation digitization is not merely advantageous but essential for ensuring compliance and operational efficiency in the pharmaceutical sector. Additionally, 46% of small enterprises squander time each day due to paper-heavy procedures, underscoring the shortcomings of conventional record-keeping methods.
Moreover, 59% of CIOs cited as essential for innovation, reinforcing the argument for adopting modern document management systems. A sample ROI calculation for a 100-employee organization reveals potential savings of $5,300 monthly, demonstrating the financial viability of implementing a DMS while ensuring expert guidance and support from AVS Life Sciences in navigating the complexities of (CSV). This method not only enhances record-keeping practices but also aligns with effective technical writing and in a virtual company.
Facilitate Training and Change Management for Successful Digitization
The successful implementation of strategies necessitates that organizations invest in designed to enhance both technical skills and compliance awareness. A needs evaluation is essential to identify among employees regarding new digital tools and methods. Tailored training modules must encompass critical topics such as , electronic signatures, and , ensuring that employees are well-equipped to navigate the .
Fostering a culture of open communication is vital to mitigate resistance to change. Encouraging feedback from employees throughout the transition process enables organizations to address concerns promptly and effectively. Implementing a structured , such as Prosci's ADKAR model, facilitates this transition by focusing on the key elements of awareness, desire, knowledge, ability, and reinforcement.
By prioritizing training and adopting robust change management strategies, organizations can significantly enhance , ensuring that their digitization efforts lead to improved compliance and operational efficiency. This approach not only addresses employee knowledge gaps but also aligns with the of the organization, ultimately driving successful outcomes in the validation documentation digitization.

Conclusion
Mastering the digitization of validation documentation is essential for organizations striving for compliance success in a highly regulated environment. Transitioning from paper-based processes to digital systems not only enhances operational efficiency but also aligns with the stringent regulatory requirements imposed by authorities, including the FDA and ISO standards. By comprehending these frameworks and implementing effective digitization strategies, organizations can ensure that their documentation processes are both compliant and robust.
This article has examined the critical importance of understanding regulatory requirements, the necessity of adopting effective digitization strategies, and the vital role of training and change management in this transition. Organizations must:
- Evaluate their current documentation practices
- Invest in suitable digital tools
- Prioritize data integrity to meet compliance standards
Furthermore, fostering a culture of continuous learning and open communication can significantly enhance employee engagement and facilitate a smoother transition to digital workflows.
Ultimately, the shift towards validation documentation digitization is not merely a trend; it is an essential step for organizations aiming for compliance and operational excellence in the pharmaceutical sector. Embracing modern document management solutions and investing in comprehensive training can lead to substantial benefits, including improved accuracy, reduced costs, and enhanced collaboration. As the landscape of regulatory compliance continues to evolve, organizations must remain proactive in adopting these best practices to secure their success in the years to come.
Frequently Asked Questions
What are the key regulatory frameworks for validation documentation?
The key regulatory frameworks for validation documentation include Good Manufacturing Practices (GMP), ISO standards, and Quality System Regulations (QSR).
Why is validation documentation digitization important?
Validation documentation digitization is important to ensure that manufacturing processes consistently produce products that meet established quality standards as mandated by GMP.
What should organizations do to ensure compliance with validation documentation regulations?
Organizations should conduct a meticulous review of relevant regulations and guidelines from authorities such as the FDA and EMA to ensure compliance.
What resources can help organizations understand digital verification requirements?
The ISPE Good Practice Guide is a valuable resource that offers insights tailored to digital verification requirements.
How can a deep understanding of regulations benefit organizations?
A deep understanding of regulations can help organizations formulate an effective compliance strategy that aligns with regulatory expectations, enhancing their operational integrity and product quality.
