Master U.S. Cosmetics Legislation 2025: Key Changes and Compliance

Overview
The article delineates the pivotal changes and compliance requirements arising from the U.S. cosmetics legislation scheduled to take effect in 2025, with a particular focus on the Modernization of Cosmetics Regulation Act (MoCRA). It underscores the imperative for cosmetic companies to:
- Register with the FDA
- Furnish comprehensive ingredient disclosures
- Comply with newly established safety standards
These measures are designed to bolster consumer protection and enhance industry transparency, as evidenced by the prescribed compliance steps and relevant state-level regulations.
Introduction
The landscape of cosmetics regulation in the U.S. is undergoing a seismic shift, driven by the introduction of the Modernization of Cosmetics Regulation Act (MoCRA) and anticipated changes set for 2025. This evolving framework not only aims to bolster consumer safety and transparency but also compels industry players to reevaluate their compliance strategies in light of stricter requirements.
As the stakes rise, the pressing question emerges: how will companies adapt to these new regulations while maintaining consumer trust and ensuring product safety in an increasingly scrutinized market? To navigate this complex environment, organizations must develop robust compliance solutions that address these challenges head-on, fostering a culture of accountability and transparency.
By engaging with experts in the field, companies can leverage best practices and case studies that exemplify successful compliance projects, ultimately reinforcing their commitment to safety and trust. The time to act is now; as regulations tighten, proactive measures will distinguish industry leaders from laggards.
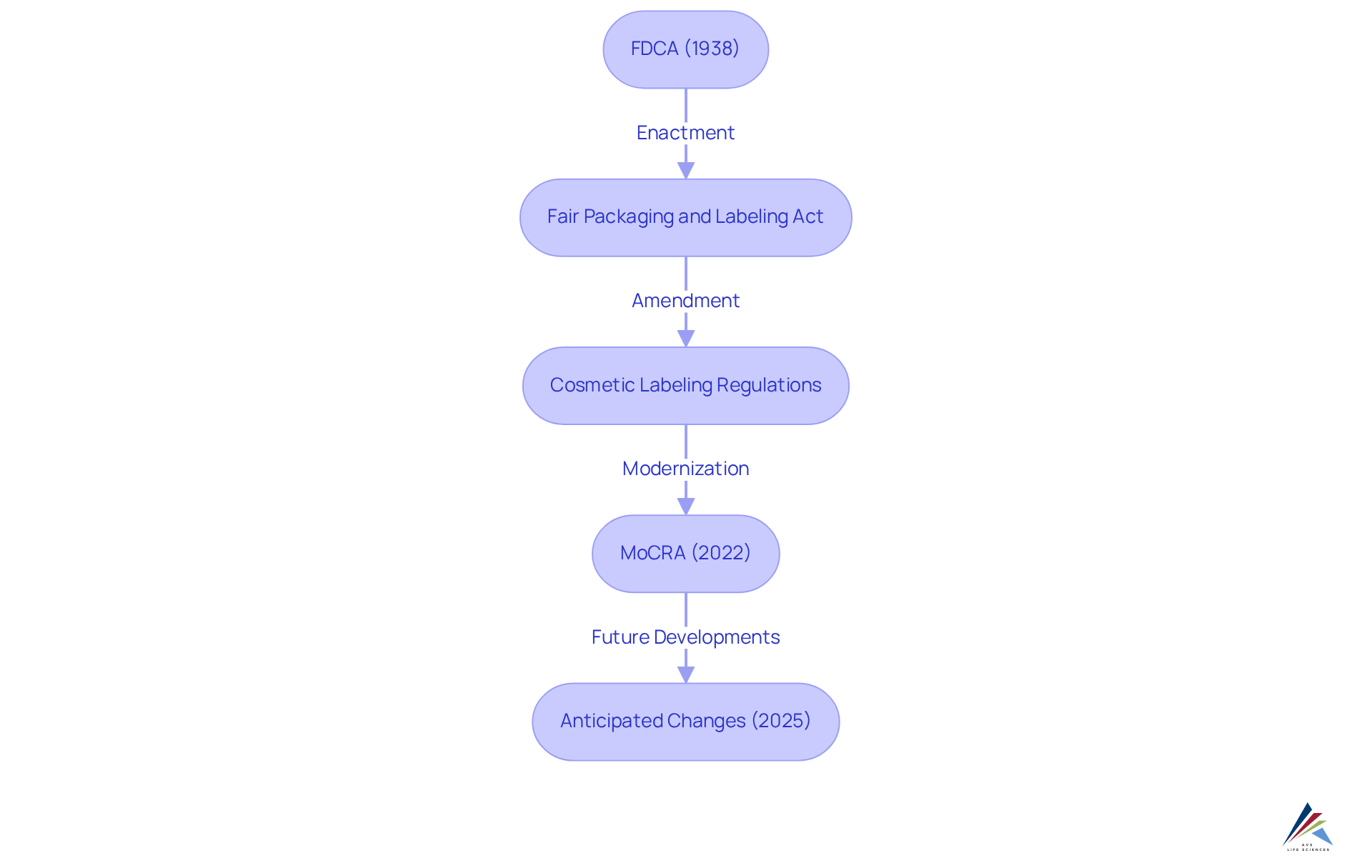
Overview of U.S. Cosmetics Legislation: Key Concepts and Historical Context
The U.S. cosmetics industry has been under [regulatory oversight since the enactment of the Federal Food, Drug, and Cosmetic Act](https://theregreview.org/2025/06/28/seminar-a-new-era-of-cosmetics-safety-regulation) (FDCA) in 1938. This landmark legislation empowered the FDA to ensure the , mandating that products be safe for public use and accurately labeled. Over the years, the regulatory landscape has evolved through various amendments, including:
- The Fair Packaging and Labeling Act
- The Cosmetic Labeling Regulations
These amendments have further clarified labeling requirements and consumer protections. For industry experts, understanding these historical milestones is crucial for navigating the complexities of regulations and standards in today's market.
The launch of the Modernization of Cosmetics Regulation Act (MoCRA) in 2022 marks a significant transformation, enhancing the FDA's regulatory authority and establishing new requirements for consumer protection and transparency. These developments lay the groundwork for substantial changes anticipated in the U.S. cosmetics legislation 2025, underscoring the necessity for ongoing vigilance in compliance practices.
Moreover, as privacy policies become increasingly significant, they may influence consumer trust in cosmetic products, highlighting the need for transparency not only in product safety but also in the management of consumer data.
2025 Legislative Changes: Impacts of the Modernization of Cosmetics Regulation Act
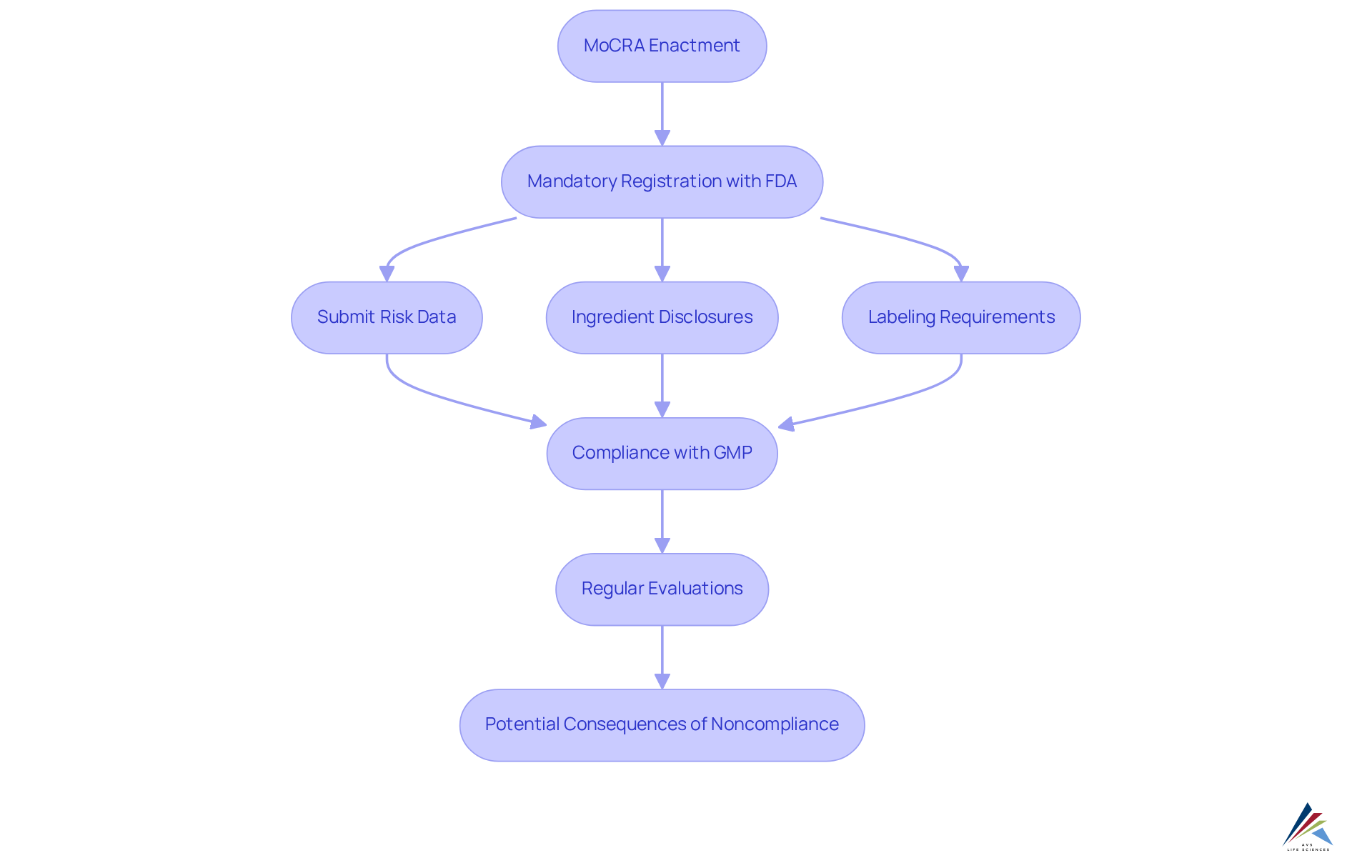
The Modernization of Cosmetics Regulation Act (MoCRA) introduces transformative changes to the cosmetics landscape as part of the U.S. cosmetics legislation 2025, set to take effect in 2025. A fundamental aspect of this legislation is the mandatory registration with the FDA, requiring all cosmetic creators to submit detailed risk data, including ingredient disclosures and allergen information. This shift not only enhances consumer protection but also increases industry transparency, prompting companies to reassess their compliance strategies.
Under MoCRA, the FDA gains and conduct more frequent evaluations, underscoring the necessity for stringent adherence to quality standards. The act mandates that all cosmetic items display the contact information of a designated 'Responsible Person' on their labels by December 29, 2024, ensuring accountability in safety.
As of January 1, 2025, the U.S. cosmetics legislation 2025 reveals that there are over 9,500 active facility registrations and nearly 590,000 item listings, highlighting the extent of compliance required. Companies must also prepare for the forthcoming U.S. cosmetics legislation 2025, which includes Good Manufacturing Practices (GMP), with final rules anticipated by December 2025. Noncompliance with these new requirements may result in severe consequences, including product seizures and legal actions.
AVS Life Sciences offers comprehensive quality management and regulatory adherence solutions that can assist companies in adapting to these changes. Our expertise in developing robust data protocols and ensuring accurate ingredient disclosures can help brands navigate the complexities of MoCRA compliance. Additionally, we provide guidance on implementing standardized testing techniques, particularly for talc-containing products, to mitigate legal risks and enhance public trust.
Case studies demonstrate the challenges faced by cosmetics companies in conforming to these regulations. Many brands are revising their manufacturing and testing processes to align with the new FDA guidelines. This proactive approach not only minimizes legal risks but also positions brands as leaders in consumer protection and trust.
In summary, the implementation of MoCRA necessitates a strategic overhaul of regulatory practices within the cosmetics industry, particularly to align with the U.S. cosmetics legislation 2025, which emphasizes the importance of safety substantiation and transparent labeling to meet the evolving regulatory landscape. AVS Life Sciences is dedicated to supporting companies through this transition, ensuring they effectively meet the new standards.
Navigating Compliance: Essential Requirements for Industry Professionals
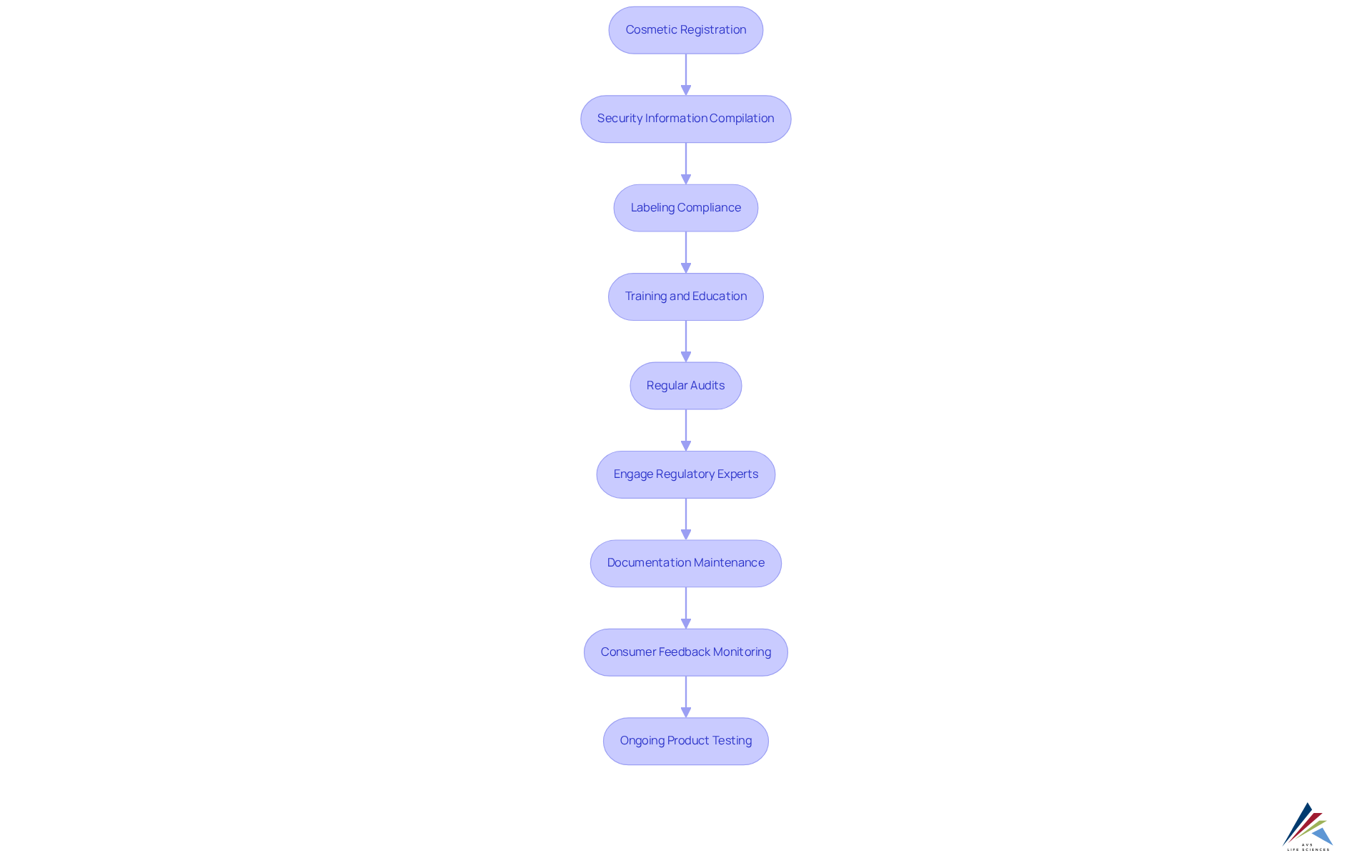
To effectively navigate the regulatory landscape shaped by U.S. cosmetics legislation, industry professionals must undertake essential steps to ensure compliance:
- Cosmetic Registration: It is imperative to register all cosmetics with the FDA prior to market entry, as mandated by the Modernization of Cosmetics Regulation Act (MoCRA). AVS Life Sciences can streamline this process with tailored consulting solutions.
- Security Information Compilation: This necessitates assembling and maintaining comprehensive security data for all components, including toxicological profiles and usage levels, to demonstrate product safety and compliance. Our experts are equipped to guide you in developing robust safety data documentation.
- Labeling Compliance: This necessitates , ensuring that all ingredients are accurately listed in descending order of concentration and that potential allergens are clearly highlighted. AVS Life Sciences offers assistance to ensure your labeling meets all standards.
- Training and Education: Implement comprehensive training programs for staff regarding adherence practices, fostering a culture of safety and accountability within your organization. AVS Life Sciences offers customized training solutions.
- Regular Audits: Conduct audits to assess adherence to the legislation and identify areas for improvement, proactively addressing potential regulatory issues. Our consulting services include audit preparation and execution.
- Engage Regulatory Experts: Seek advice from specialists to ensure complete and compliant registration or notification under U.S. cosmetics legislation. AVS Life Sciences has a team of regulatory specialists ready to assist you.
- Documentation Maintenance: Maintain detailed documentation of formulation, testing, and manufacturing processes as required for registration. Our consultants can help you establish effective documentation practices.
- Consumer Feedback Monitoring: Regularly monitor consumer feedback for adverse reactions or product issues as part of post-market surveillance. AVS Life Sciences can assist in setting up effective feedback mechanisms.
- Ongoing Product Testing: This is necessary to ensure continuous evaluations for ongoing reliability and effectiveness. Our experts can guide you in establishing a comprehensive testing protocol.
By following these steps and leveraging the expertise of AVS Life Sciences, companies can maintain compliance with U.S. cosmetics legislation and mitigate the risk of legal challenges.
State Regulations and Initiatives: Enhancing Federal Standards in Cosmetics Safety
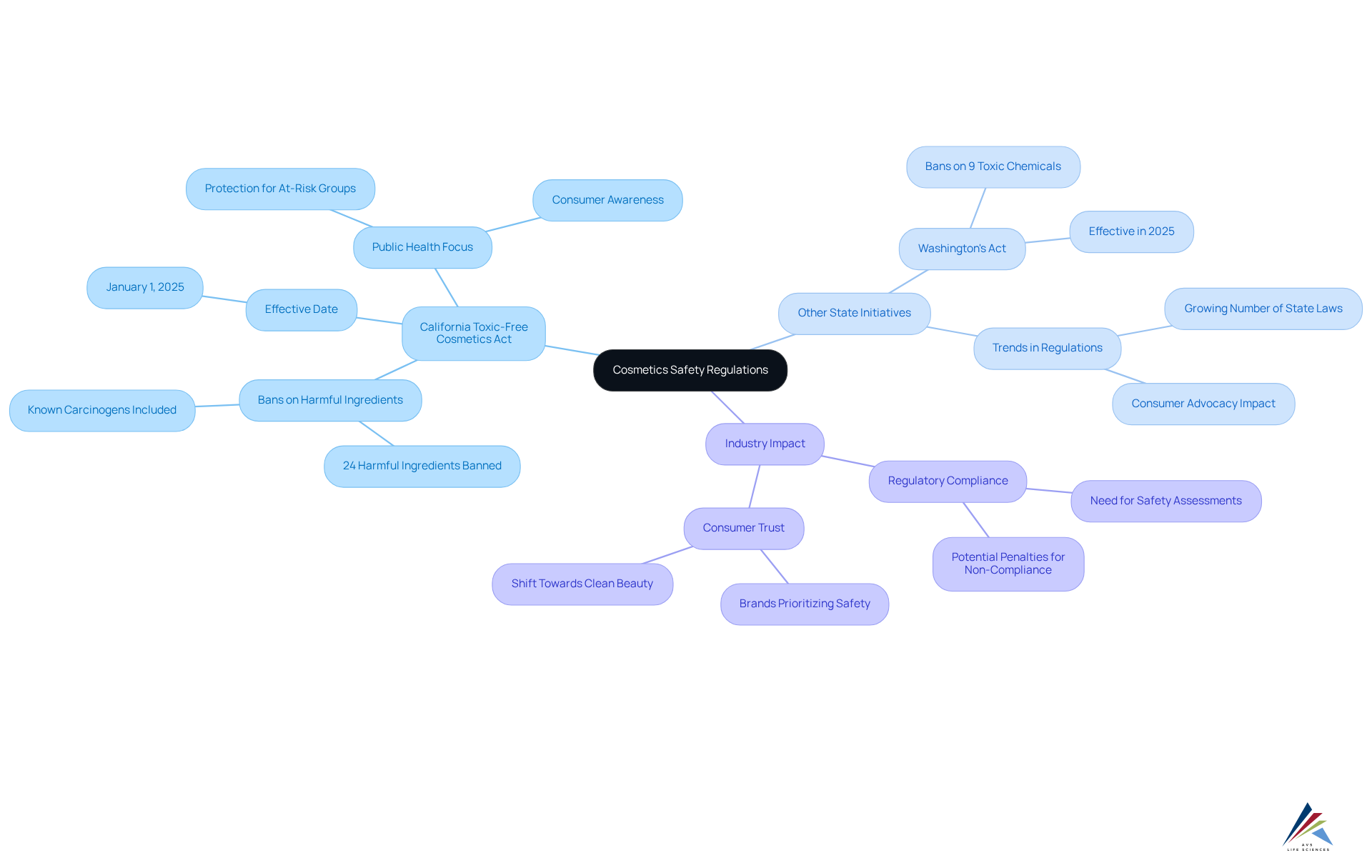
In addition to federal regulations, several states have enacted their own laws to enhance cosmetics safety, with California leading the charge through its Toxic-Free Cosmetics Act. This landmark bans 24 harmful ingredients, including known carcinogens like formaldehyde and PFAS, and it will be effective starting January 1, 2025. The measure seeks to safeguard individuals, especially at-risk groups, from the negative health impacts linked to harmful substances in personal care items. For instance, women use an average of 13 personal care products daily, while men use an average of 11 products containing 105 unique ingredients, underscoring the need for stringent regulations.
California's initiative has set a precedent, inspiring similar measures in other states, such as Washington's Toxic-Free Cosmetics Act, which prohibits nine toxic chemicals and is in line with U.S. cosmetics legislation 2025. These state-level initiatives demonstrate a rising trend towards stricter regulations and public protection, highlighting the significance of transparency and accountability in the beauty sector.
Industry leaders have praised California's Toxic-Free Cosmetics Act as a significant step forward for public health. They emphasize that adherence to such regulations is not merely a legal duty but also a moral necessity for brands seeking to establish trust with consumers. Significantly, 65% of firms prioritize profit over safety assessments, emphasizing the need for regulatory adherence. Companies must stay informed about these laws, as non-compliance can lead to substantial penalties and reputational damage. It is advisable for businesses to conduct a thorough review of state regulations in all markets where they operate and adjust their compliance strategies accordingly to ensure adherence to both federal and state standards.
Conclusion
The forthcoming changes to U.S. cosmetics legislation in 2025, primarily propelled by the Modernization of Cosmetics Regulation Act (MoCRA), herald a significant transformation in the operational dynamics of the cosmetics industry. Enhanced regulatory oversight and stringent compliance requirements necessitate that companies prioritize safety and transparency to align with both consumer expectations and legal mandates.
Key insights from this article underscore the imperative for cosmetic manufacturers to:
- Register their products with the FDA
- Maintain comprehensive safety data
- Adhere to updated labeling standards
The emergence of state-level regulations, such as California's Toxic-Free Cosmetics Act, further accentuates the critical nature of compliance across various jurisdictions. These developments are designed not only to safeguard consumers but also to challenge brands to cultivate a culture of accountability and trust.
As the cosmetics landscape evolves, it is essential for industry professionals to adopt a proactive stance in adjusting to these changes. Embracing compliance mitigates legal risks and positions brands as frontrunners in consumer safety and ethical practices. Engaging with experts and implementing robust regulatory strategies will be vital for navigating the complexities of the new legislation and ensuring a successful transition into 2025 and beyond.
Frequently Asked Questions
What is the primary legislation governing cosmetics in the U.S.?
The primary legislation governing cosmetics in the U.S. is the Federal Food, Drug, and Cosmetic Act (FDCA), enacted in 1938.
What powers does the FDA have under the FDCA?
The FDCA empowers the FDA to ensure the safety of cosmetics, mandating that products be safe for public use and accurately labeled.
What are some key amendments to cosmetics legislation in the U.S.?
Key amendments include the Fair Packaging and Labeling Act and the Cosmetic Labeling Regulations, which clarify labeling requirements and enhance consumer protections.
What is the Modernization of Cosmetics Regulation Act (MoCRA)?
The Modernization of Cosmetics Regulation Act (MoCRA), launched in 2022, enhances the FDA's regulatory authority and establishes new requirements for consumer protection and transparency in the cosmetics industry.
What changes are anticipated in U.S. cosmetics legislation by 2025?
Substantial changes are anticipated in U.S. cosmetics legislation by 2025, which will require ongoing vigilance in compliance practices.
How do privacy policies relate to the cosmetics industry?
Privacy policies are becoming increasingly significant as they may influence consumer trust in cosmetic products, highlighting the need for transparency in both product safety and the management of consumer data.
