Master Third Party Verification: Steps for Compliance Officers

Overview
The article delineates crucial steps for compliance officers to excel in third-party verification, highlighting the necessity of:
- Grasping industry regulations
- Preparing requisite documentation
- Executing the verification process
- Addressing common challenges
It substantiates this by outlining specific:
- Regulatory frameworks
- Documentation practices
- Proactive strategies to mitigate potential issues
Thereby ensuring strict adherence to compliance standards within the pharmaceutical sector.
Introduction
Navigating the complexities of third-party verification is paramount for compliance officers in the pharmaceutical industry, where adherence to stringent regulations can significantly impact a company's reputation. This guide serves as a roadmap for mastering the verification process, ensuring that organizations not only fulfill regulatory requirements but also bolster their operational integrity. As the landscape evolves with new standards on the horizon, compliance officers must confront the inherent challenges of verification head-on, all while upholding compliance and quality control.
Understand Third-Party Verification Requirements
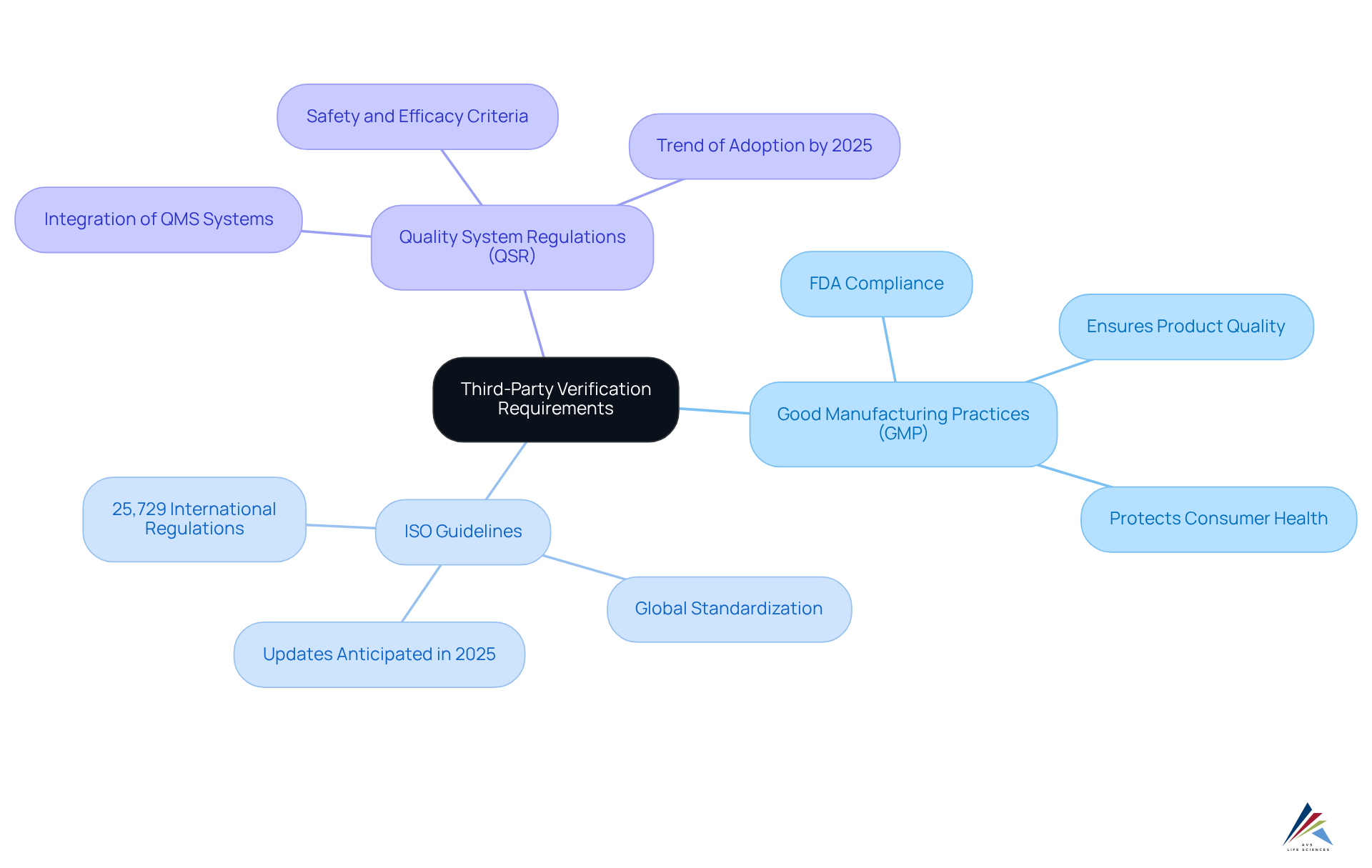
To effectively navigate the process of , regulatory officers must familiarize themselves with the specific requirements relevant to their industry. This involves a comprehensive understanding of the following regulations:
- : These guidelines ensure that products are consistently produced and controlled according to established quality standards, thereby safeguarding consumer health and ensuring compliance with regulatory requirements. Compliance with GMP regulations is mandatory for all , as stated by the FDA.
- : These international criteria are essential for ensuring quality, safety, and efficiency across products, services, and systems. ISO has established 25,729 international regulations, emphasizing the extent of its impact and the significance of these guidelines in the pharmaceutical sector. Major updates are anticipated in 2025 that will affect regulatory strategies.
- : These regulations govern the quality management systems specifically for medical devices, ensuring that they meet stringent safety and efficacy criteria. By 2025, over 85% of pharmaceutical companies will have integrated QMS systems, emphasizing the growing trend in the industry.
Compliance officers should diligently examine documentation supplied by and industry organizations. This fundamental knowledge is crucial for directing subsequent steps in the , allowing organizations to uphold high levels of adherence and quality control. Furthermore, it is crucial to acknowledge the challenges encountered in this field, as 48% of organizations indicate difficulties in monitoring third party verification compliance.
Prepare Necessary Documentation and Data
Once the requirements are understood, the next step is to gather and prepare all necessary documentation and data. This typically includes:
- : Ensure that all QMS documents are up-to-date and reflect current practices, adhering to GXP standards and FDA regulations.
- : Gather prior audit reports, along with any corrective actions implemented, to demonstrate adherence to internal and external auditing techniques.
- : Compile records that showcase compliance with relevant regulations, such as training records and equipment calibration logs, ensuring alignment with .
- : Compile documentation related to third-party suppliers, including contracts and performance evaluations, as part of the to maintain quality management in your supply chain.
Arranging these documents in an orderly manner will facilitate a seamless review process. Consider utilizing a document management system to effectively monitor all files and ensure easy access during the validation.

Execute the Third-Party Verification Process
With documentation ready, the next step is to carry out the . This involves:
- Selecting a : Choose an auditor with relevant experience and credentials in your industry. Look for auditors who have a proven track record in and understand the specific of your sector.
- Scheduling the Assessment: Collaborate with the auditor to establish a date for the assessment process, ensuring that all necessary personnel are available. It is recommended to arrange the assessment well ahead of time, as the average duration for third-party evaluation in life sciences can differ, often necessitating several weeks to finalize.
- : During the review process, the auditor will examine documentation, carry out interviews, and perform site inspections as necessary. Ensure that all staff are prepared to provide information and answer questions. Effective communication during this phase is vital, as it can greatly influence the result of the assessment.
- : Following the assessment, the auditor will offer a report outlining any findings. Address these findings promptly by implementing and documenting the steps taken. Industry specialists highlight that prompt reactions to audit discoveries are crucial for upholding regulations and fostering trust with regulatory authorities.
Adhering to these steps will assist in guaranteeing a and uphold alignment with .

Troubleshoot Common Verification Challenges
Despite meticulous preparation, compliance officers often encounter challenges during the third party verification assessment process. Key issues include:
- Incomplete Documentation: Ensuring that all required documents are complete and readily accessible is crucial. When gaps are identified, prompt action must be taken to gather the necessary information. Compliance officers should emphasize the importance of to prevent delays in the assessment. AVS Life Sciences offers extensive that optimize documentation workflows, ensuring all essential files are in order.
- : When auditors raise concerns or discrepancies, it is imperative to address these issues without delay. Providing additional evidence or clarification can help resolve misconceptions and uphold the integrity of the . AVS Life Sciences delivers that prepare compliance officers to respond effectively to auditor inquiries.
- Staff Unpreparedness: Regular training sessions for personnel involved in the validation process are essential. Ensuring team members understand their roles and responsibilities will enhance the accuracy of the information provided and streamline the . AVS Life Sciences offers designed to equip staff with the necessary knowledge and skills for successful validation.
- : In the event of setbacks during the validation process, proactive communication with auditors is vital. Keeping all stakeholders informed and rescheduling as necessary can mitigate the impact of these delays. AVS Life Sciences underscores the importance of and can assist in developing strategies to manage timelines effectively.
By anticipating these challenges and implementing effective strategies—leveraging AVS Life Sciences' expertise—compliance officers can significantly enhance the efficiency of the verification process through third party verification and ensure successful outcomes.

Conclusion
Mastering third-party verification is essential for compliance officers who aim to uphold regulatory standards and ensure product quality in the pharmaceutical industry. A comprehensive understanding of specific requirements and regulations, such as GMP, ISO guidelines, and QSR, forms the foundation for effective compliance. This expertise, coupled with meticulous preparation of necessary documentation and a structured verification process, empowers organizations to maintain their commitment to quality and safety.
The article delineates key steps in the third-party verification process, including:
- Gathering of essential documentation
- Executing thorough assessments with qualified auditors
- Addressing common challenges that may arise
By proactively preparing for potential obstacles—such as incomplete documentation, auditor discrepancies, and staff unpreparedness—compliance officers can enhance the efficiency of the verification process and foster trust with regulatory authorities.
Ultimately, the significance of third-party verification is paramount. It not only ensures compliance with industry regulations but also safeguards consumer health and confidence in pharmaceutical products. By implementing best practices and leveraging available resources, compliance officers can adeptly navigate the complexities of third-party verification, paving the way for a successful and compliant operation that meets the evolving demands of the industry.
Frequently Asked Questions
What is the importance of understanding third-party verification requirements?
Understanding third-party verification requirements is crucial for regulatory officers to navigate compliance effectively within their industry, ensuring adherence to established quality standards and safeguarding consumer health.
What are Good Manufacturing Practices (GMP)?
Good Manufacturing Practices (GMP) are guidelines that ensure products are consistently produced and controlled according to quality standards. Compliance with GMP is mandatory for all pharmaceutical manufacturers as mandated by the FDA.
How many ISO regulations exist, and what is their significance?
There are 25,729 international ISO regulations that are essential for ensuring quality, safety, and efficiency across products, services, and systems. These guidelines have a significant impact on the pharmaceutical sector, with major updates expected in 2025.
What are Quality System Regulations (QSR)?
Quality System Regulations (QSR) govern the quality management systems specifically for medical devices, ensuring they meet stringent safety and efficacy criteria.
What trend is expected regarding Quality Management Systems (QMS) in the pharmaceutical industry by 2025?
By 2025, over 85% of pharmaceutical companies are expected to have integrated Quality Management Systems (QMS), indicating a growing trend towards enhanced quality management in the industry.
What challenges do organizations face regarding third-party verification compliance?
Organizations face significant challenges in monitoring third-party verification compliance, with 48% indicating difficulties in this area.
Why is it important for compliance officers to examine documentation from regulatory agencies?
It is important for compliance officers to examine documentation from regulatory agencies and industry organizations to ensure they have the fundamental knowledge necessary to direct subsequent steps in the verification process and maintain high levels of adherence and quality control.
