Master the Toxic-Free Beauty Act: Compliance Strategies for Officers

Overview
The Toxic-Free Beauty Act is a pivotal legislation designed to eliminate harmful substances from beauty products. By banning 18 hazardous chemicals, it promotes consumer safety and transparency in the beauty industry.
Manufacturers face significant compliance challenges; thus, the article delineates effective strategies to navigate these complexities. Emphasizing the necessity of:
- Thorough ingredient reviews
- Meticulous documentation
- Regular audits
It highlights how these practices can mitigate substantial legal and financial risks associated with non-compliance. Engaging with AVS Life Sciences offers manufacturers the opportunity to align with these compliance solutions, ensuring adherence to the Act while fostering a commitment to consumer safety.
Introduction
The introduction of the Toxic-Free Beauty Act signifies a pivotal transformation in the beauty industry, responding to escalating concerns regarding the safety of personal care products that are often filled with harmful chemicals. This legislation is designed not only to safeguard consumers by prohibiting 18 hazardous substances but also to empower them through mandated transparency on product labels.
Nonetheless, as compliance becomes increasingly intricate, critical questions emerge:
- How can manufacturers and compliance officers adeptly navigate this new legal landscape while preserving their brands and maintaining consumer trust?
Overview of the Toxic-Free Beauty Act: Legislative Context and Objectives
The toxic-free beauty act, established in 2025, represents a significant legislative initiative aimed at eliminating harmful substances from beauty and personal care products. This initiative arises from increasing public concern regarding the safety of beauty items, particularly those containing dangerous substances linked to serious health risks such as cancer and reproductive harm. The Act specifically targets 18 hazardous chemicals, including mercury, parabens, and formaldehyde, as well as entire classes of chemicals like phthalates. By implementing stringent regulations, the Act seeks to protect consumers, especially vulnerable groups such as women of color and salon workers, who are at greater risk of exposure to these toxic substances.
The objectives of the toxic-free beauty act are unequivocal: to enhance consumer safety and promote transparency within the beauty industry. This legislation not only seeks to ban harmful chemicals but also mandates the disclosure of hazardous materials on product labels, empowering consumers to make informed choices. Notably, the Act allocates $30 million for research and public education on product safety, emphasizing its dedication to consumer awareness. Public concern about the safety of beauty products is underscored by statistics indicating that the average American adult uses approximately 12 personal care items daily, leading to exposure to around 168 distinct chemicals.
Case studies illustrate the Act's potential impact on beauty manufacturers, showcasing how compliance with these new regulations can transform product formulations and marketing strategies. Furthermore, the Cosmetic Hazardous Ingredient Right to Know Act, which is part of the legislative framework, requires the disclosure of hazardous fragrance and flavor ingredients, further bolstering consumer protection. As the industry adapts to these changes, the Act is anticipated to cultivate a safer beauty landscape, aligning U.S. standards more closely with those of the European Union, which has already prohibited many of these harmful chemicals. Understanding the is essential for regulatory officers to effectively navigate this evolving legal landscape and ensure consumer safety.

Key Compliance Requirements of the Toxic-Free Beauty Act
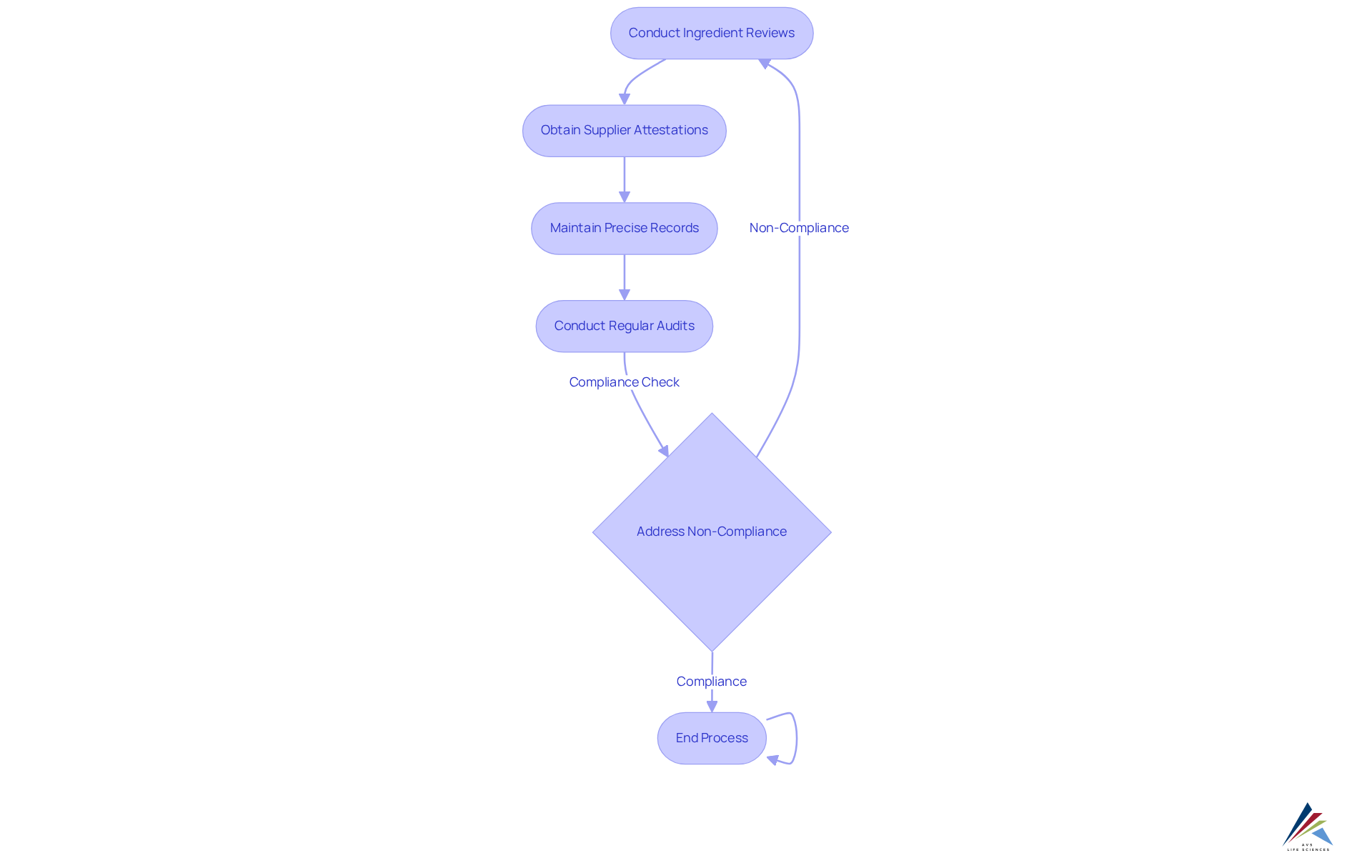
Adherence to the toxic-free beauty act imposes several essential requirements for manufacturers, distributors, and retailers of cosmetic items. Companies must conduct comprehensive ingredient reviews to ensure that none of the 18 banned chemicals or prohibited classes are present in their formulations. This process involves obtaining supplier attestations that confirm the absence of these substances. Moreover, companies must keep precise records of their adherence efforts, which encompass safety data sheets and comprehensive formulations. Regular audits are crucial to to the toxic-free beauty act, and any instances of non-compliance must be addressed swiftly to mitigate potential penalties.
In 2025, it is estimated that only 40% of manufacturers are fully conducting ingredient reviews for adherence, underscoring the need for enhanced diligence in this area. Compliance officers must understand these requirements to create effective strategies that conform to the law, ensuring the safety and transparency of beauty products in accordance with the toxic-free beauty act. Furthermore, the temporary policy on lead in cosmetics, which permits short-term adherence flexibility, highlights the changing regulatory environment that regulatory officers must navigate.
AVS Life Sciences provides thorough quality management and regulatory adherence solutions that can help companies fulfill these requirements efficiently. By partnering with AVS Life Sciences, organizations can enhance their compliance processes, ensuring they are well-equipped to meet the evolving standards of the beauty industry.
Implementing Compliance Strategies: Best Practices for Documentation and Auditing
To effectively implement adherence strategies to the , businesses must adopt several best practices.
- Establish a centralized documentation system that tracks all compliance-related materials, including ingredient lists, supplier communications, and audit reports. This system should be easily accessible to relevant stakeholders.
- Conduct regular internal audits to evaluate adherence to the Act, concentrating on ingredient sourcing and product formulations. These audits must be documented thoroughly, with findings and corrective actions recorded for future reference.
- Provide instruction for staff on regulatory obligations and best practices, fostering a culture of accountability within the organization.
Notably, firms that conduct organized internal audits demonstrate a 30% increase in adherence effectiveness, underscoring the significance of these strategies. Furthermore, it is essential to recognize the specific harmful chemicals banned under the Toxic-Free Beauty Act, including PFAS, lead, and formaldehyde, as these regulations aim to protect vulnerable populations, particularly women of color. By adhering to these practices, compliance officers can ensure their companies remain aligned with the Toxic-Free Beauty Act, ultimately promoting safer products for consumers.

Consequences of Non-Compliance: Risks and Legal Implications
Non-compliance with the toxic-free beauty act poses significant legal and financial risks for businesses. Companies found in violation of the Act may incur civil penalties that can reach thousands of dollars per infraction. In 2025, the Consumer Product Safety Commission (CPSC) projected civil penalties totaling 28.28 million USD, highlighting the substantial financial stakes involved.
Additionally, non-compliance can result in:
- Product recalls
- Damage to brand reputation
- Erosion of consumer trust
Legal actions may also be initiated by consumers or advocacy groups aiming to hold companies accountable for failing to meet safety standards. For instance, the recent recalls of over 80 million beauty units due to safety concerns are part of a broader context, wherein the FDA reported 334 recalls of dermatological items affecting more than 77 million units over the past 12 years.
Furthermore, ongoing non-compliance can attract increased scrutiny from regulatory bodies, leading to more frequent inspections and audits. As Tracy Y. Williams, an attorney, emphasizes, "Any business that manufactures, distributes, sells, or uses cosmetic products in Washington must comply with the TFCA." Recognizing these risks is crucial for compliance officers, as it enables them to and safeguard their organizations from potential repercussions.

Conclusion
The Toxic-Free Beauty Act signifies a crucial advancement in consumer safety within the beauty industry by eradicating harmful chemicals from personal care products. This legislation not only prohibits specific hazardous substances but also requires transparency in product labeling, thus empowering consumers to make informed choices. As compliance officers navigate this evolving legal landscape, grasping the Act's implications is vital for cultivating a safer beauty environment.
Key points discussed encompass:
- The rigorous compliance requirements imposed on manufacturers
- The necessity of thorough documentation and auditing practices
- The severe repercussions of non-compliance, which can result in significant financial penalties and reputational harm
With statistics revealing that numerous companies are still falling short in adherence, adopting best practices for compliance is imperative for safeguarding consumers, particularly vulnerable populations at heightened risk from toxic exposure.
Ultimately, the Toxic-Free Beauty Act serves as a poignant reminder of the beauty industry's obligation to prioritize consumer safety. By embracing compliance strategies and nurturing a culture of accountability, companies can not only avert legal repercussions but also contribute to a healthier and more transparent beauty landscape. The call to action is unequivocal: prioritize compliance to protect both consumers and the integrity of the beauty industry in a swiftly evolving regulatory environment.
Frequently Asked Questions
What is the Toxic-Free Beauty Act?
The Toxic-Free Beauty Act, established in 2025, is a legislative initiative aimed at eliminating harmful substances from beauty and personal care products to enhance consumer safety.
Why was the Toxic-Free Beauty Act created?
The Act was created in response to increasing public concern over the safety of beauty products that contain dangerous substances linked to serious health risks, such as cancer and reproductive harm.
What specific chemicals does the Toxic-Free Beauty Act target?
The Act specifically targets 18 hazardous chemicals, including mercury, parabens, and formaldehyde, as well as entire classes of chemicals like phthalates.
Who are the vulnerable groups that the Act aims to protect?
The Act aims to protect consumers, particularly vulnerable groups such as women of color and salon workers, who are at greater risk of exposure to toxic substances.
What are the main objectives of the Toxic-Free Beauty Act?
The main objectives are to enhance consumer safety, promote transparency in the beauty industry, ban harmful chemicals, and mandate the disclosure of hazardous materials on product labels.
How much funding does the Act allocate for research and public education?
The Act allocates $30 million for research and public education on product safety.
What is the significance of the average American adult's use of personal care items in relation to the Act?
The average American adult uses approximately 12 personal care items daily, leading to exposure to around 168 distinct chemicals, highlighting the need for safer products.
How might the Toxic-Free Beauty Act impact beauty manufacturers?
The Act may transform product formulations and marketing strategies as manufacturers comply with new regulations aimed at ensuring consumer safety.
What additional legislation supports the goals of the Toxic-Free Beauty Act?
The Cosmetic Hazardous Ingredient Right to Know Act, which requires the disclosure of hazardous fragrance and flavor ingredients, further supports consumer protection.
How does the Toxic-Free Beauty Act align U.S. standards with those of the European Union?
The Act is anticipated to align U.S. standards more closely with the European Union, which has already prohibited many harmful chemicals in beauty products.
