Master the New Drug Application Process in 4 Steps

Overview
The new drug application (NDA) process represents a structured four-step approach:
- Preclinical research
- IND application submission
- Clinical trials
- Post-marketing surveillance
This process is essential for securing FDA approval for new medications. Thorough documentation and efficient communication with the FDA throughout these stages are paramount to addressing common challenges and enhancing the likelihood of successful approvals. This underscores the complexity and significant investment involved in drug development, highlighting the necessity for compliance solutions that can navigate these intricate requirements effectively.
Introduction
The journey of bringing a new medication to market is a complex and rigorous process, with the New Drug Application (NDA) serving as the crucial gateway to regulatory approval. Pharmaceutical companies must navigate a labyrinth of preclinical research, clinical trials, and comprehensive documentation to demonstrate their drug's safety and efficacy. However, with only a fraction of candidates successfully making it through this arduous process, the stakes are high.
What strategies can companies employ to not only master the NDA process but also enhance their chances of approval in an increasingly competitive landscape? Understanding these challenges is vital for success, as the landscape of pharmaceutical compliance continues to evolve.
Understand the New Drug Application (NDA) Process
The new drug application (NDA) process represents a formal proposal submitted to the FDA by pharmaceutical companies aiming to secure approval for marketing a new medication. This process encompasses several essential steps that are pivotal for compliance and regulatory success:
- Preclinical Research: Prior to NDA submission, companies conduct extensive laboratory and animal studies to gather data on the medication's safety and effectiveness. This foundational research is crucial for informing subsequent phases, ensuring a robust basis for the application.
- Investigational New Drug (IND) Application: Before initiating clinical trials, an IND application must be submitted and approved. This application includes data from preclinical studies and outlines the proposed clinical trial plan, ensuring that the FDA is informed of the intended research, thereby facilitating a smoother approval process.
- Clinical Trials: The NDA procedure consists of three stages of clinical trials (Stage 1, Stage 2, and Stage 3) designed to assess the treatment's safety and efficacy in humans. Notably, Phase 3 trials can enroll hundreds to thousands of patients, typically lasting about 3.3 years and costing tens to hundreds of millions of dollars, underscoring the complexity and investment involved in this stage.
- Following successful clinical trials, the new drug application is submitted, containing comprehensive data from the trials, proposed labeling, and manufacturing information. The FDA's review of the new drug application is critical, with average timelines for review in 2025 expected to range from 6 to 12 months, depending on the complexity of the new drug application and whether it qualifies for priority review.
- The FDA conducts a thorough examination of the new drug application to ensure the medication is safe and effective for its intended use. This rigorous review process reflects the , especially considering that only about 12% of substances entering clinical trials ultimately receive FDA approval.
- Post-Marketing Surveillance: Once the medication is approved, it enters the market; however, ongoing monitoring for safety and efficacy continues through Phase 4 trials and adverse event reporting. This phase is essential for identifying any rare or long-term side effects that may not have been apparent during earlier trials.
As the pharmaceutical environment evolves, firms are increasingly leveraging advanced technologies such as AI to optimize the new drug application process and enhance data analysis. This innovation holds the potential to significantly reduce both the time and costs associated with medication development, ultimately benefiting compliance and regulatory outcomes.
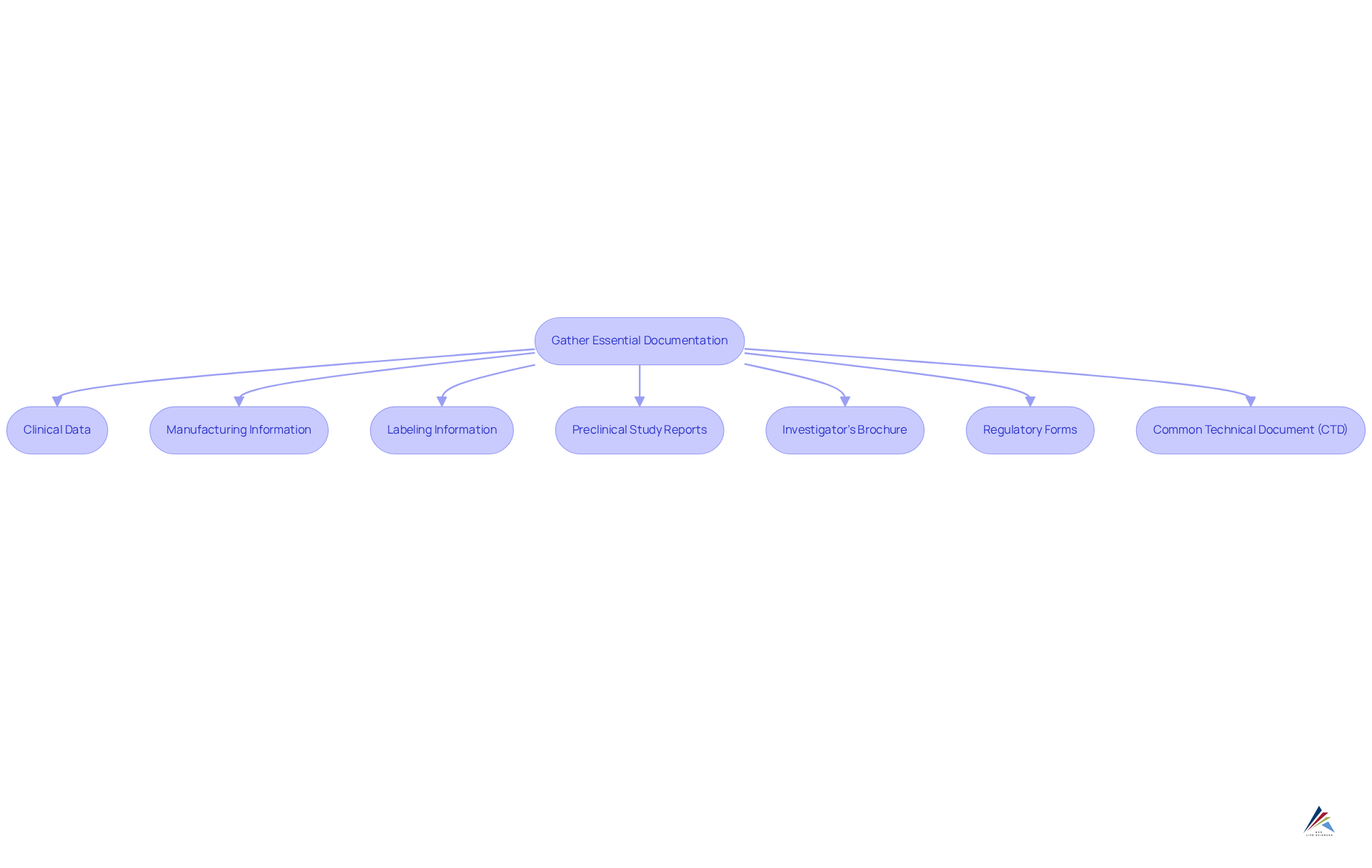
Gather Essential Documentation and Requirements
To prepare for an NDA submission, it is imperative to gather a comprehensive set of documents and data, including:
- Clinical Data: Compile and present results from all clinical trials, encompassing safety and efficacy data clearly.
- Manufacturing Information: Provide detailed information about the medication's manufacturing process, quality control measures, and adherence to Good Manufacturing Practices (GMP).
- Labeling Information: Include proposed labeling that covers indications, dosage, administration, and safety information.
- Preclinical Study Reports: Summarize laboratory and animal studies that support the drug's safety profile.
- Investigator's Brochure: Prepare a document that equips clinical investigators with the necessary information to conduct the trial.
- Regulatory Forms: Ensure all forms are complete and accurate, including Form FDA 356h, the official request for NDA approval.
- Common Technical Document (CTD): Arrange your application in accordance with the CTD format, which is an internationally acknowledged standard for NDA applications.
Moreover, it is essential to verify that all data is validated prior to transmission, as emphasized in the new drug application Data Submission Agreement. Regulatory advisors often face challenges, such as ensuring adherence to the latest guidelines and avoiding excessive data collection that can lead to rejections. Incorporating insights from experts, such as Richard L. Schilsky, who emphasizes the importance of gathering essential safety information over excessive mild adverse event data, can significantly enhance the credibility of your proposal. Staying updated on new drug application submission requirements is crucial, especially with the evolving landscape in 2025, to bolster your chances of a successful new drug application.
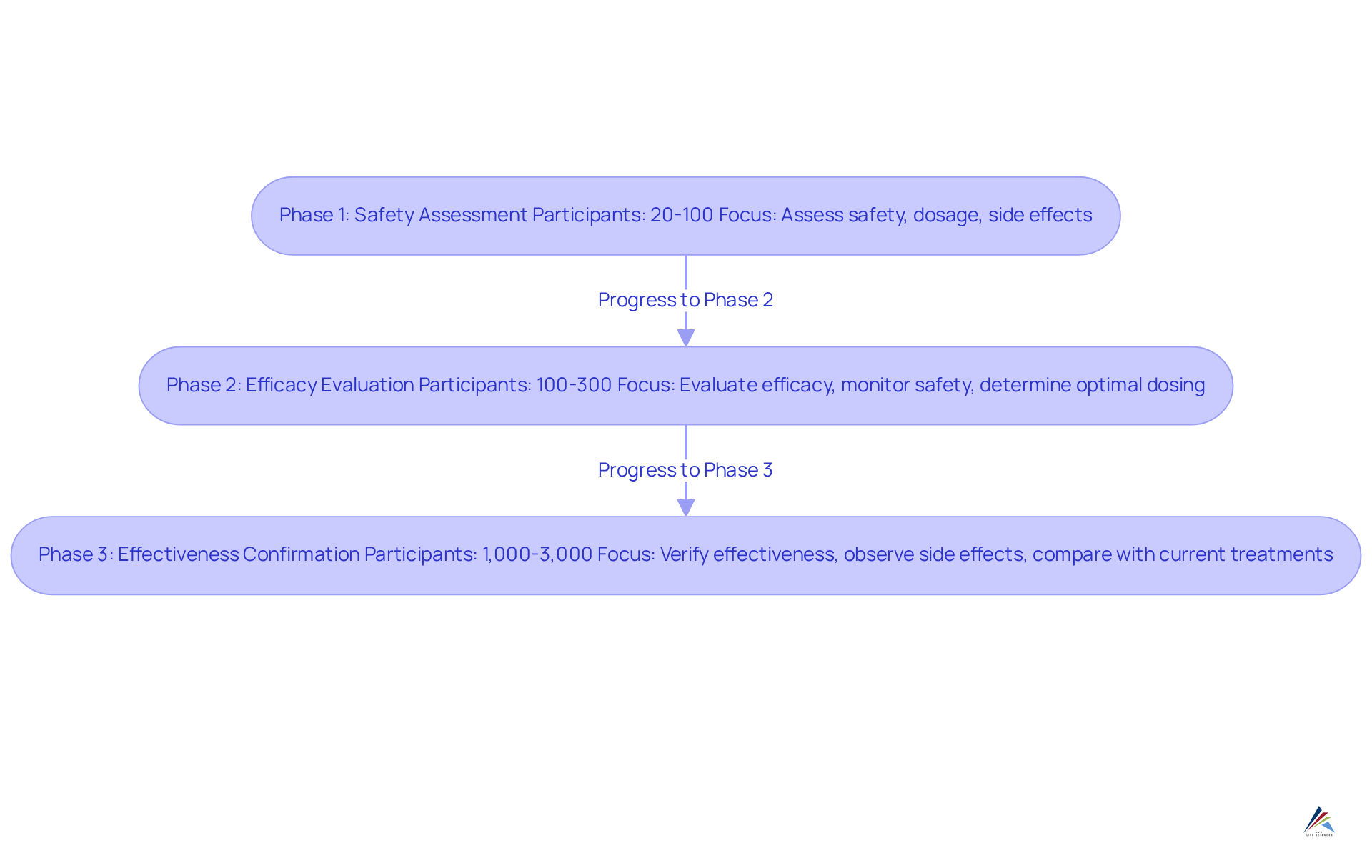
Complete Required Clinical Trials Phases
The clinical trial process is meticulously structured into three critical phases, each serving distinct purposes in the drug development journey:
- Phase 1: This initial phase typically involves a small cohort of healthy volunteers, ranging from 20 to 100 participants. The main aim is to assess the medication's safety profile, establish the suitable dosage range, and recognize any possible side effects. This phase is crucial for understanding how the medication is metabolized and its pharmacokinetics, laying the groundwork for subsequent trials. Notably, about 63%-70% of medications successfully progress through Stage 1, underscoring the significance of careful preparation and implementation.
- Phase 2: In this stage, the medication is administered to a larger group of patients, typically between 100 and 300, who have the condition the treatment aims to address. The focus shifts to evaluating the substance's efficacy while continuing to monitor its safety. Key objectives include determining the optimal dosing regimen and collecting preliminary data on the medication's effectiveness, which is vital for informing the new drug application. For instance, successful Stage 2 results can greatly influence the chances of the new drug application (NDA) approval, as they provide crucial information on the treatment's therapeutic advantages.
- Stage 3: This stage broadens the participant pool considerably, encompassing 1,000 to 3,000 patients. The main goal is to verify the medication's effectiveness, observe side effects, and compare its performance against current treatments. Successful fulfillment of Stage 3 is essential for producing the robust data necessary for the new drug application, as it offers extensive insights into the medication's therapeutic advantages and safety profile.
Understanding the from Phase 1 to Phase 3 is vital; roughly 30% of substances entering Phase 1 progress to Phase 2, and around 58% of those in Phase 2 advance to Phase 3. This highlights the importance of meticulous planning and execution at each stage to enhance the likelihood of regulatory approval. Engaging with clinical trial experts can further optimize these processes, ensuring adherence to best practices and improving overall outcomes. Additionally, it is important to acknowledge that the average time-to-market for drugs exceeds 10 years, emphasizing the lengthy and complex nature of drug development.
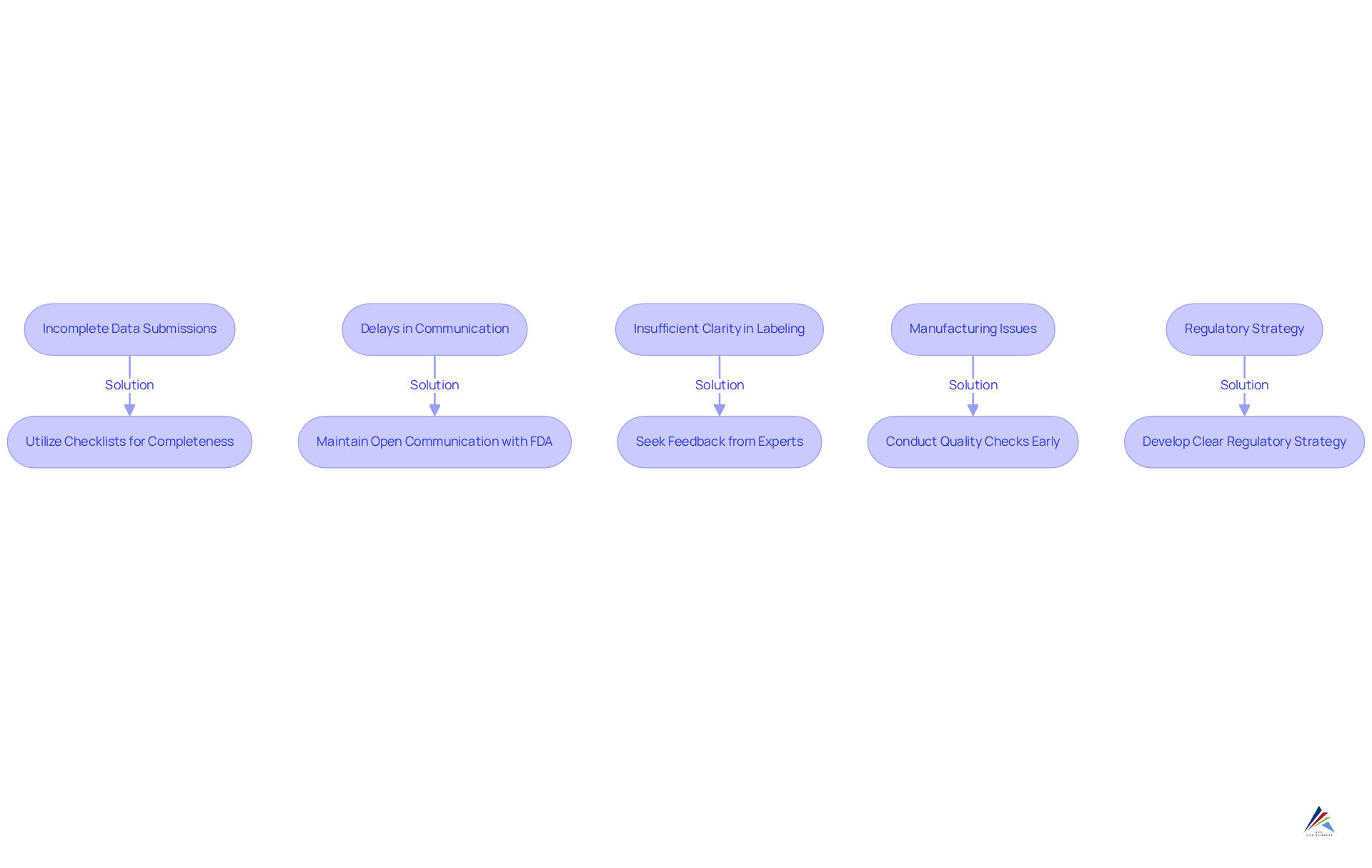
Troubleshoot Common NDA Challenges
Navigating the new drug application process can present various challenges that require strategic solutions. Here are some common issues and effective strategies to troubleshoot them:
- Incomplete Data Submissions: It is imperative to ensure that all required data from clinical trials is included and well-organized. Utilizing checklists can help verify completeness and prevent omissions that could delay the review process.
- Delays in Communication with the FDA: Maintaining open lines of communication with the FDA is crucial. Regularly checking for updates and responding promptly to inquiries can significantly impact the timelines of the new drug application. Companies that are proactive in their communication often experience smoother interactions and quicker resolutions. Engaging in pre-NDA discussions with the FDA can also elucidate filing strategies and expectations, further improving communication.
- Insufficient Clarity in Labeling: The proposed labeling must be clear and comprehensive. Seeking feedback from regulatory consultants or legal experts can enhance clarity and ensure compliance with FDA expectations, thereby reducing the likelihood of requests for revisions.
- Manufacturing Issues: Addressing any manufacturing concerns early in the process is essential. Compliance with Good Manufacturing Practices (GMP) is critical, and conducting thorough quality checks can help identify potential issues before they escalate.
- Regulatory Strategy: Developing a clear regulatory strategy from the outset, including timelines and milestones, is vital. This proactive method aids in maintaining the on schedule and allows for necessary modifications, ensuring that all elements of the application conform to FDA requirements. Understanding the FDA's review timelines—approximately 10 months for standard drugs and 6 months for priority drugs—underscores the urgency of addressing these challenges.
Moreover, partnering with Contract Research Organizations (CROs) can offer valuable assistance in post-approval monitoring and compliance reporting, significantly increasing the chances of a successful new drug application. As compliance officers note, effective communication and thorough preparation are key to overcoming NDA submission hurdles. AVS Life Sciences exemplifies this commitment to excellence, having achieved zero findings during audits, which showcases effective compliance practices.
Conclusion
Mastering the new drug application (NDA) process is crucial for pharmaceutical companies aiming to introduce innovative medications to market. This structured approach not only ensures compliance with regulatory standards but also significantly enhances the likelihood of approval. By grasping the intricacies of each step—from preclinical research to post-marketing surveillance—companies can navigate the complexities of drug development more effectively.
This article outlines the essential phases of the NDA process, emphasizing the importance of thorough documentation, rigorous clinical trials, and proactive communication with regulatory bodies. Each stage, including:
- Preclinical research
- IND application
- The three phases of clinical trials
plays a pivotal role in establishing a drug's safety and efficacy. Additionally, addressing common challenges such as:
- Incomplete data submissions
- Manufacturing issues
is key to ensuring a smooth application process.
Ultimately, the NDA process is a vital component of drug approval that requires meticulous planning, comprehensive data collection, and ongoing collaboration with the FDA. Embracing advanced technologies and strategic regulatory planning can significantly streamline this journey. For those involved in pharmaceutical development, staying informed about evolving requirements and best practices will not only enhance compliance but also contribute to the successful introduction of new therapies that can improve patient outcomes.
Frequently Asked Questions
What is the New Drug Application (NDA) process?
The NDA process is a formal proposal submitted to the FDA by pharmaceutical companies to secure approval for marketing a new medication. It involves several essential steps for compliance and regulatory success.
What is involved in preclinical research before submitting an NDA?
Preclinical research includes extensive laboratory and animal studies to gather data on the medication's safety and effectiveness, providing a robust basis for the NDA application.
What is the purpose of the Investigational New Drug (IND) application?
The IND application must be submitted and approved before initiating clinical trials. It includes data from preclinical studies and outlines the proposed clinical trial plan, informing the FDA of the intended research.
How many stages are there in clinical trials during the NDA process?
There are three stages of clinical trials (Stage 1, Stage 2, and Stage 3) designed to assess the treatment's safety and efficacy in humans.
What is the duration and cost of Phase 3 clinical trials?
Phase 3 trials can enroll hundreds to thousands of patients, typically lasting about 3.3 years and costing tens to hundreds of millions of dollars.
What does the NDA submission contain?
The NDA submission contains comprehensive data from clinical trials, proposed labeling, and manufacturing information.
How long does the FDA review the new drug application?
The FDA's review of the NDA typically takes between 6 to 12 months, depending on the complexity of the application and whether it qualifies for priority review.
What percentage of substances entering clinical trials receive FDA approval?
Only about 12% of substances entering clinical trials ultimately receive FDA approval.
What happens after a medication is approved?
Once approved, the medication enters the market, but ongoing monitoring for safety and efficacy continues through Phase 4 trials and adverse event reporting.
How are advanced technologies impacting the NDA process?
Pharmaceutical firms are increasingly using advanced technologies such as AI to optimize the NDA process and enhance data analysis, potentially reducing the time and costs associated with medication development.
