Master the FSP Model: Key Insights for Compliance Officers

Overview
The FSP model in clinical research serves as a strategic outsourcing framework, empowering pharmaceutical companies to collaborate effectively with specialized service providers for the management of specific clinical study functions. This collaboration significantly enhances operational efficiency and compliance. By allowing sponsors to retain oversight while leveraging external expertise, the model ultimately leads to improved study outcomes and substantial cost savings in an increasingly complex clinical research environment. Such a framework addresses the pressing compliance challenges faced by the industry, providing a robust solution that fosters both accountability and innovation.
Introduction
The landscape of clinical research is evolving rapidly, with the FSP model emerging as a pivotal strategy for pharmaceutical companies and research organizations. This innovative outsourcing framework allows sponsors to harness specialized expertise while maintaining critical oversight of their clinical studies.
As organizations increasingly seek to navigate the complexities of modern trials, compliance officers face pressing challenges:
- How can they effectively implement and manage the FSP model to enhance operational efficiency?
- How can they ensure regulatory compliance?
This article delves into the key insights, benefits, and challenges of the FSP model, providing essential guidance for compliance professionals aiming to optimize their clinical research strategies. By exploring successful compliance projects and industry standards, we aim to equip readers with the knowledge necessary to drive impactful change within their organizations.
Define the FSP Model in Clinical Research
The FSP model in clinical research represents a strategic outsourcing framework where pharmaceutical companies or research organizations collaborate with specialized service providers to manage specific functions of clinical studies. This framework not only allows sponsors to maintain oversight but also leverages the expertise of external partners for critical tasks such as:
By outsourcing these functions, organizations can significantly enhance operational efficiency, thereby enabling them to focus on their core competencies. This approach not only leads to improved study outcomes but also ensures adherence to , an increasingly vital requirement in today's complex clinical study landscape.
Recent trends indicate a growing reliance on the FSP model, especially as the complexities of clinical studies increase, driven by advancements in personalized medicine and biologics. The integration of AI and automation tools further streamlines these processes, enhancing data accuracy and compliance.
As the global clinical research outsourcing market is projected to reach USD 86.81 billion by 2033, the FSP model is being increasingly preferred by clients who are seeking flexibility and specialized expertise in their clinical operations.
Explain How the FSP Model Operates
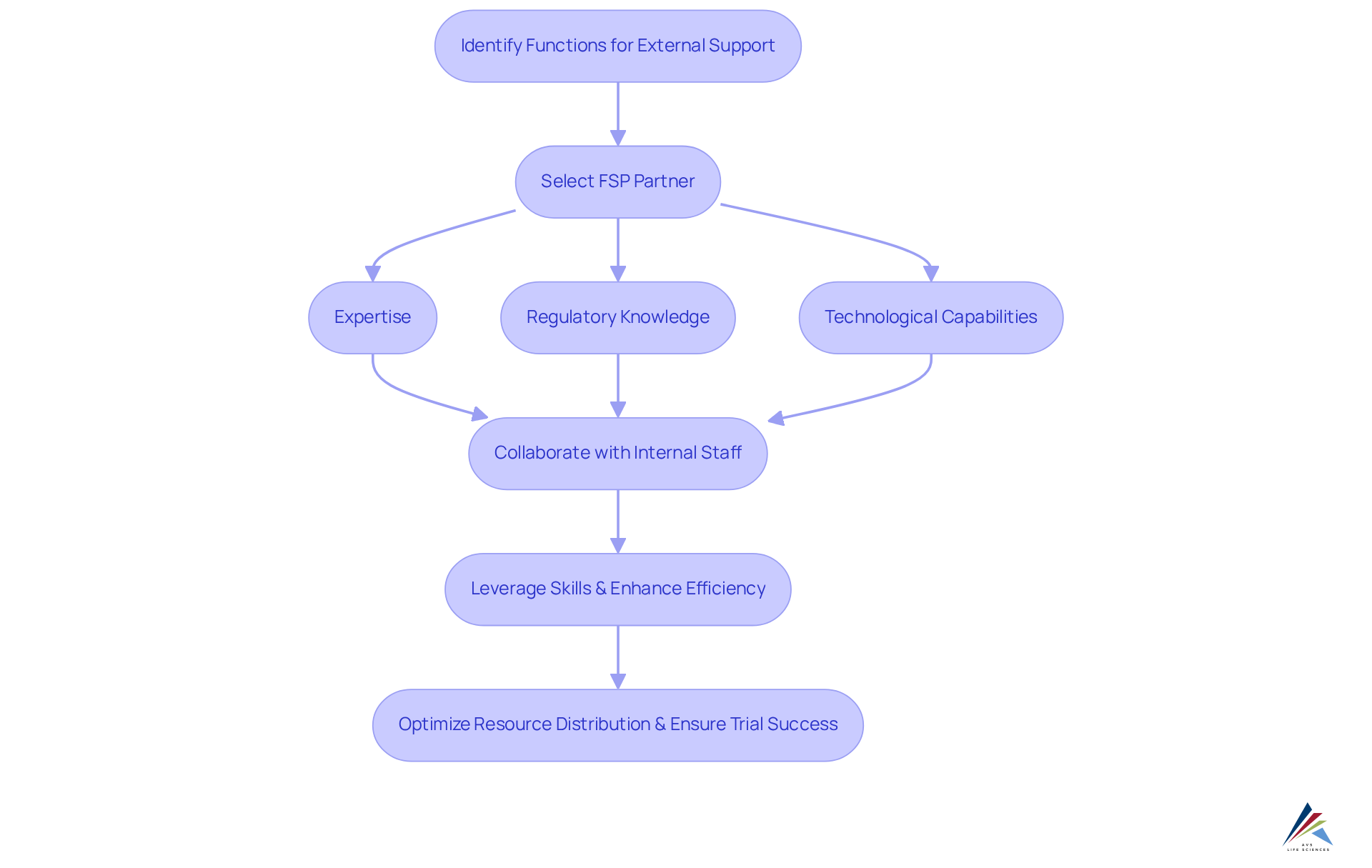
The fsp model empowers funders to strategically outsource specific clinical trial functions—such as biostatistics, data management, and clinical monitoring—while retaining oversight and control over the entire trial process. Initially, backers must identify the functions that necessitate external support. Subsequently, they select an FSP partner based on critical criteria, including:
Upon selection, the FSP team collaborates closely with the backer's internal staff, fostering seamless cooperation and communication. This collaboration not only allows supporters to leverage specialized skills and resources but also enhances efficiency and adherence to regulatory requirements. Notably, the adoption of the fsp model has surged, with market utilization increasing from 28% in 2018 to over 40% in 2021. This trend reflects a to navigate the complexities of clinical research. By effectively implementing the fsp model, backers can optimize resource distribution, mitigate risks associated with understaffing, and ensure the successful execution of intricate trials.
Identify the Benefits of the FSP Model
The advantages offered by the fsp model are significant for organizations engaged in clinical research.
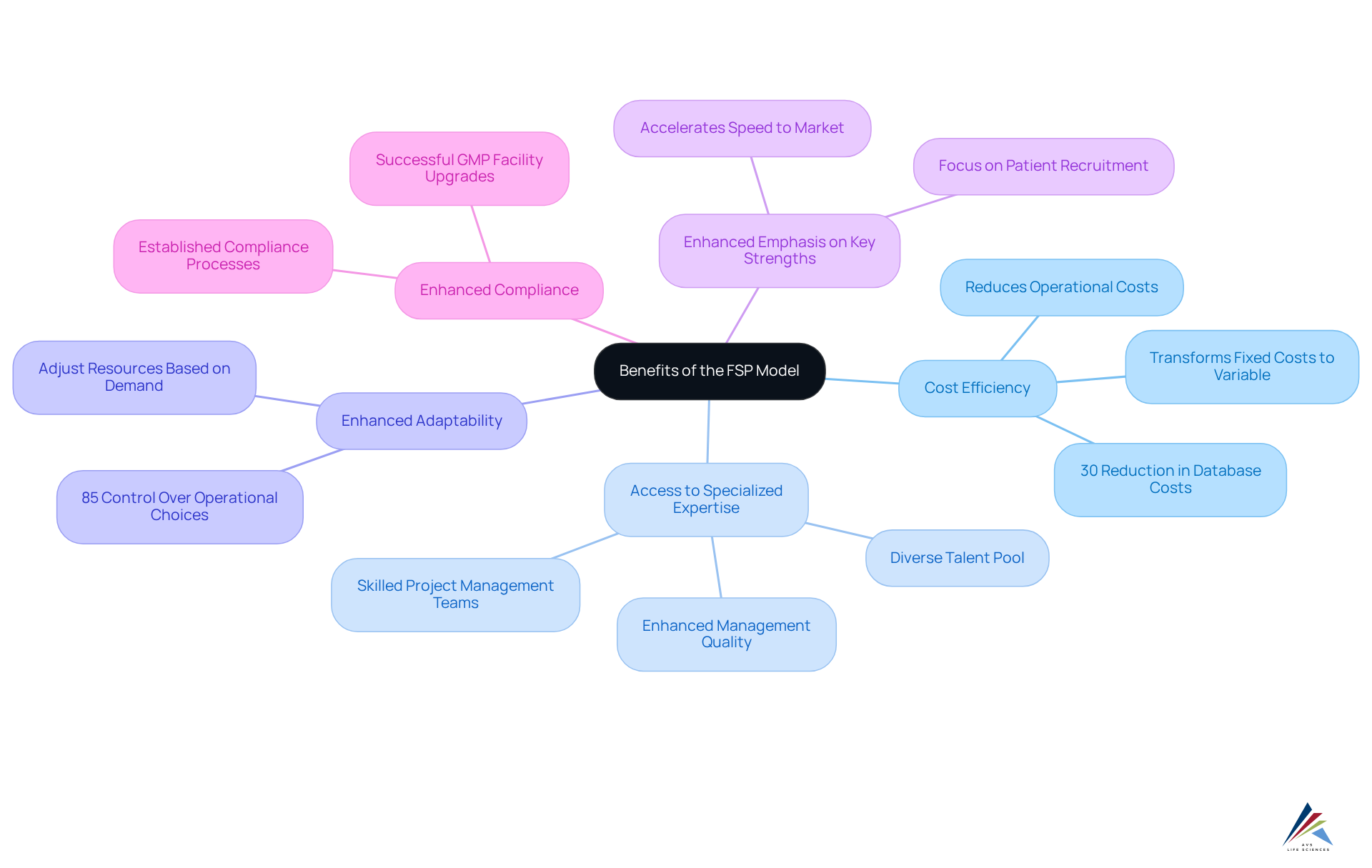
- Cost Efficiency: Outsourcing specific functions allows sponsors to significantly reduce operational costs associated with hiring and training internal staff. This system transforms fixed labor costs into variable expenses, enabling organizations to pay solely for the services they require, leading to substantial savings without sacrificing quality. Notably, the fsp model has reduced database expenses by over 30% in organization-intensive fields such as rare diseases and cell and gene therapy.
- Access to Specialized Expertise: FSPs provide sponsors with access to a diverse pool of specialized talent and knowledge that may not be available in-house. This expertise enhances management quality, particularly in complex therapeutic fields where deep understanding is essential. Additionally, FSPs offer skilled project management teams to oversee operations, further improving management quality.
- Enhanced Adaptability: The FSP framework empowers sponsors to adjust resources based on project demands, offering the responsiveness needed to address evolving project needs. Sponsors maintain direct authority over 85% of operational choices in FSP engagements, underscoring the system's adaptability and control. This flexibility is crucial for managing the intricacies of clinical studies, especially as the market is projected to exceed $32 billion by 2032, highlighting the importance of the fsp model in navigating this dynamic environment.
- Enhanced Emphasis on Key Strengths: By delegating non-essential tasks to FSPs, organizations can concentrate on strategic elements of their projects, such as patient recruitment and regulatory planning. This focus boosts productivity and accelerates the speed to market for new therapies.
- Enhanced Compliance: FSPs typically possess established processes and systems that align with regulatory requirements, aiding clients in maintaining compliance with Good Manufacturing Practices (GMP) and other standards. A notable example is AVS Life Sciences' recent collaboration with a leading biotechnology company, where they successfully upgraded a GMP facility from Level 1 to Level 2. This project not only satisfied the client's quality assurance standards but also ensured full traceability throughout the process. During this upgrade, critical lessons were learned regarding the barcode scanner issue, which highlighted the necessity for thorough testing and oversight. Such expertise in compliance and operational excellence is vital in an increasingly complex regulatory landscape, where staying ahead of changes is essential for success.
As we approach 2025, with cost optimization becoming a defining priority for clinical trial backers, the FSP model stands out for its ability to deliver both operational efficiency and specialized support, making it an essential strategy for navigating the evolving clinical research environment.
Discuss Challenges and Considerations in FSP Implementation
Implementing the FSP model presents several challenges and considerations that must be navigated effectively:
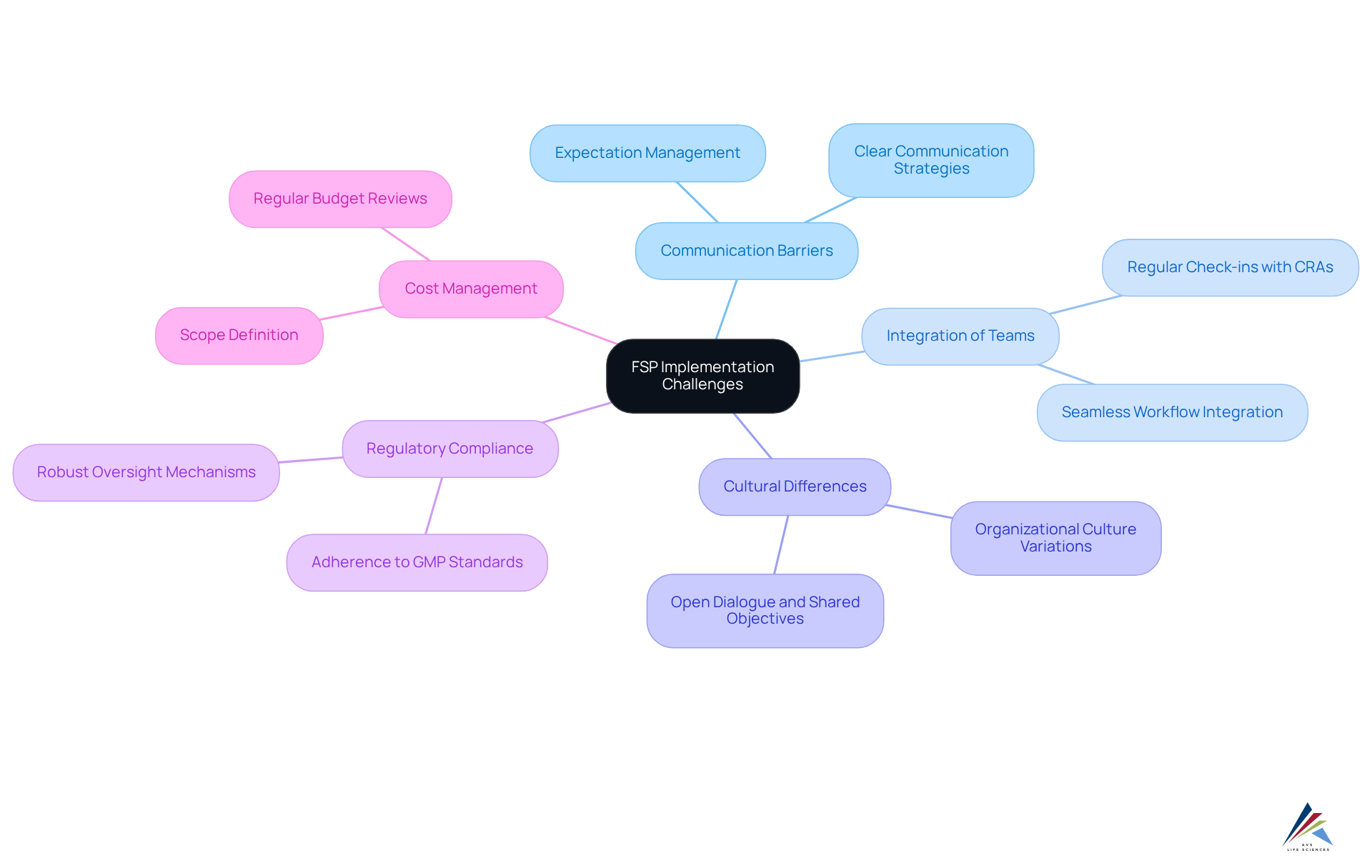
- Communication Barriers: Effective communication between backers and FSPs is paramount. Misalignment in expectations can lead to project delays and compliance issues, with nearly 90% of organizations reporting that significantly enhance their FSP engagements. Establishing clear communication expectations at the outset is essential for deriving value from FSP partnerships.
- Integration of Teams: Seamlessly integrating external teams into existing workflows requires meticulous planning and management. Frequent check-ins with Clinical Research Associates (CRAs) are crucial for tracking progress and confirming work completion as per the client's specifications, ensuring that all parties are aligned on project goals and timelines.
- Cultural Differences: Variations in organizational culture between funders and FSPs can hinder collaboration and affect project outcomes. Establishing cultural alignment through open dialogue and shared objectives is essential for fostering a productive partnership.
- Regulatory Compliance: Adherence to regulatory requirements is critical for FSPs. Sponsors must implement robust oversight mechanisms to monitor compliance, ensuring that all activities align with Good Manufacturing Practices (GMP) and other relevant standards. AVS Life Sciences emphasizes the importance of regulatory compliance by providing tailored solutions that ensure adherence to these standards throughout the project lifecycle.
- Cost Management: Although the FSP model can offer cost efficiencies, unforeseen expenses may arise if the scope of work is not clearly defined from the outset. Establishing detailed contracts and conducting regular budget reviews can help manage costs effectively and prevent budget overruns. AVS Life Sciences supports clients in this area by offering comprehensive quality management practices that help define project scopes and manage budgets efficiently.
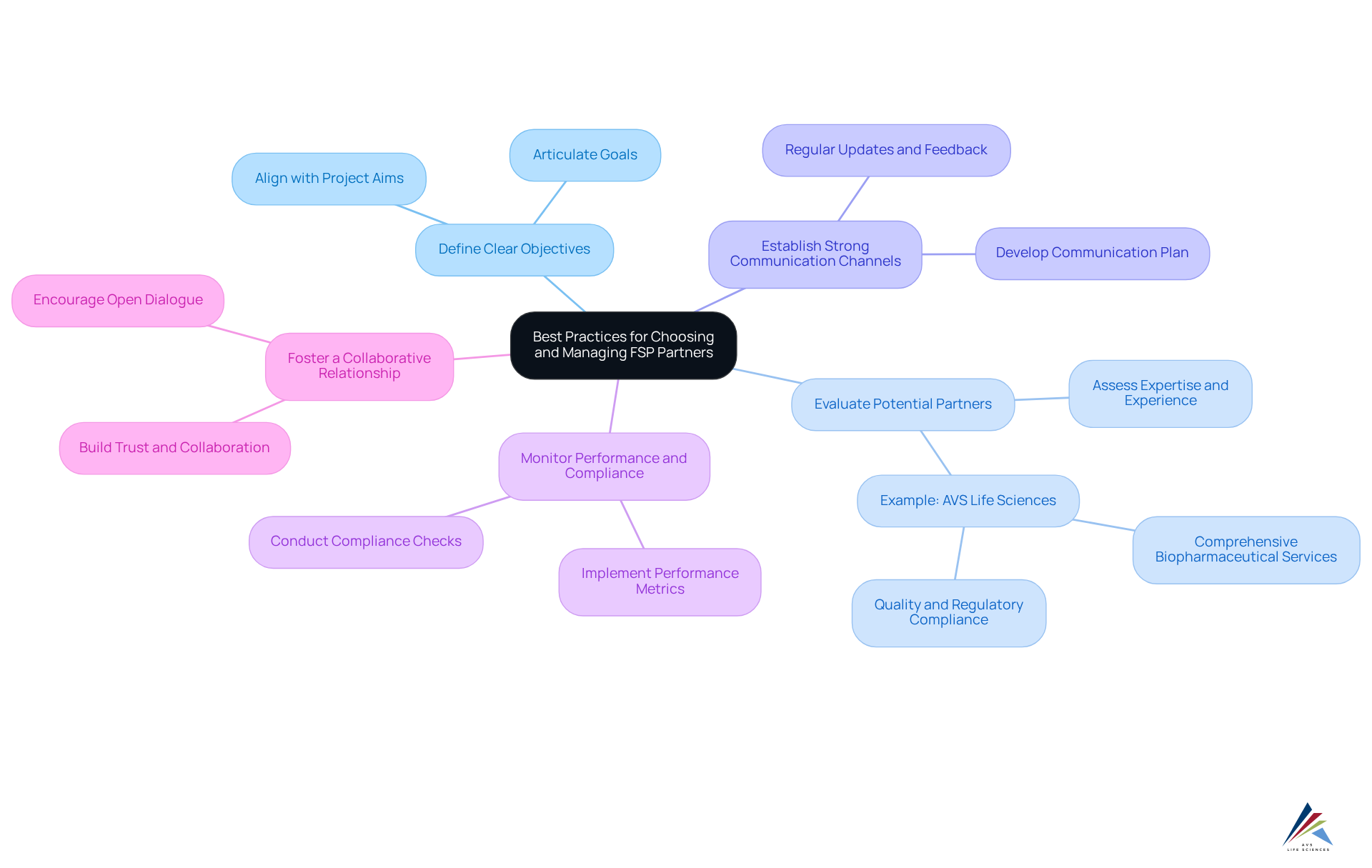
Outline Best Practices for Choosing and Managing FSP Partners
To effectively choose and manage FSP partners, compliance officers should consider the following best practices:
- Define Clear Objectives: Sponsors must articulate their goals and the specific functions they intend to outsource, ensuring alignment with overall project aims. This clarity is essential for navigating compliance challenges.
- Evaluate Potential Partners: Assess FSPs based on their expertise, experience, and proven success in relevant therapeutic areas, as this can significantly impact project outcomes. For example, AVS Life Sciences offers comprehensive biopharmaceutical services that support each stage of drug development, ensuring quality and regulatory compliance, including GXP Training and Compliance Audits & Gap Assessments.
- Establish Strong Communication Channels: Develop a comprehensive communication plan that details how information will flow between the sponsor and the FSP. This plan should include regular updates and feedback mechanisms to foster transparency, which is critical for effective collaboration.
- Monitor Performance and Compliance: Implement robust performance metrics and compliance checks to ensure the FSP adheres to agreed-upon standards and regulatory requirements. This vigilance is crucial for maintaining data integrity and operational efficiency, especially given AVS Life Sciences' throughout the drug development lifecycle.
- Foster a Collaborative Relationship: Build a partnership grounded in trust and collaboration, encouraging open dialogue and joint problem-solving to effectively navigate challenges as they arise. This approach not only enhances project execution but also aligns with the growing trend of outsourcing in the pharmaceutical sector, projected to reach USD 37.7 billion by 2034. An ideal partner for the FSP model, such as AVS Life Sciences, provides a scalable and flexible approach to meet specific needs, which is vital for successful project management.
Conclusion
The FSP model signifies a transformative approach in clinical research, empowering sponsors to optimize their operations through strategic outsourcing of specific functions while maintaining essential oversight. This framework not only enhances efficiency but also enables organizations to leverage specialized expertise, ensuring compliance with the ever-evolving regulatory landscape. As the complexities of clinical trials continue to escalate, the FSP model emerges as a critical strategy for effectively navigating these challenges.
Key insights throughout the article underscore the operational dynamics of the FSP model, highlighting its capacity to reduce costs, improve adaptability, and enhance compliance. The growing adoption of this model within the industry is indicative of its benefits, with a notable increase in market utilization reflecting the demand for flexible, expert-driven solutions. However, successful implementation necessitates a careful approach to communication strategies, team integration, cultural alignment, and robust compliance measures to mitigate potential challenges.
In light of these insights, it is imperative for compliance officers and organizations engaged in clinical research to recognize the FSP model as a strategic partner in their operations. By leveraging best practices for selecting and managing FSP partners, organizations can not only achieve operational excellence but also expedite the development of innovative therapies. Emphasizing collaboration and clear communication will ultimately empower sponsors to navigate the complexities of clinical trials, ensuring successful outcomes in an increasingly competitive landscape.
Frequently Asked Questions
What is the FSP model in clinical research?
The FSP model in clinical research is a strategic outsourcing framework where pharmaceutical companies or research organizations collaborate with specialized service providers to manage specific functions of clinical studies, such as data management, clinical monitoring, and regulatory compliance.
What are the benefits of using the FSP model?
The FSP model enhances operational efficiency, allows organizations to focus on their core competencies, improves study outcomes, and ensures adherence to regulatory standards.
Why is the FSP model becoming more popular in clinical research?
The FSP model is increasingly preferred due to the growing complexity of clinical studies, advancements in personalized medicine and biologics, and the integration of AI and automation tools that enhance data accuracy and compliance.
How does the FSP model operate?
The FSP model allows funders to outsource specific clinical trial functions while retaining oversight. Funders identify the functions needing external support, select an FSP partner based on expertise, regulatory knowledge, and technological capabilities, and collaborate with the FSP team for efficient trial execution.
What has been the trend in the adoption of the FSP model?
The adoption of the FSP model has surged, with market utilization increasing from 28% in 2018 to over 40% in 2021, reflecting a growing reliance on these partnerships to manage the complexities of clinical research.
What is the projected growth of the global clinical research outsourcing market?
The global clinical research outsourcing market is projected to reach USD 86.81 billion by 2033, indicating a significant growth trajectory for the FSP model and related services.
