Master REMS Program Drugs: Strategies for Compliance Success

Overview
The article underscores the critical role of Risk Evaluation and Mitigation Strategies (REMS) programs in safeguarding drug safety and ensuring compliance, particularly for medications that present significant safety concerns. Effective implementation of REMS necessitates clear communication, comprehensive training, and diligent monitoring to adeptly navigate the complexities of compliance. This is evident through UBC's extensive involvement in active strategies and the FDA's guidance on structured program design, which collectively illustrate the necessity for robust compliance solutions. By addressing these challenges head-on, stakeholders can foster a culture of safety and accountability within the pharmaceutical landscape.
Introduction
Risk Evaluation and Mitigation Strategies (REMS) are pivotal in the pharmaceutical landscape, acting as a crucial safeguard against the potential dangers posed by certain medications. These programs ensure that the benefits of high-risk drugs significantly outweigh their risks, while also requiring a structured approach to compliance that can be overwhelming for many organizations. As the regulatory environment continues to evolve, the challenge persists: how can pharmaceutical professionals adeptly navigate the complexities of REMS to enhance patient safety and ensure adherence? This article explores essential strategies for mastering REMS program drugs, providing insights that empower stakeholders to achieve compliance success while safeguarding public health.
Define REMS Programs and Their Importance in Drug Safety
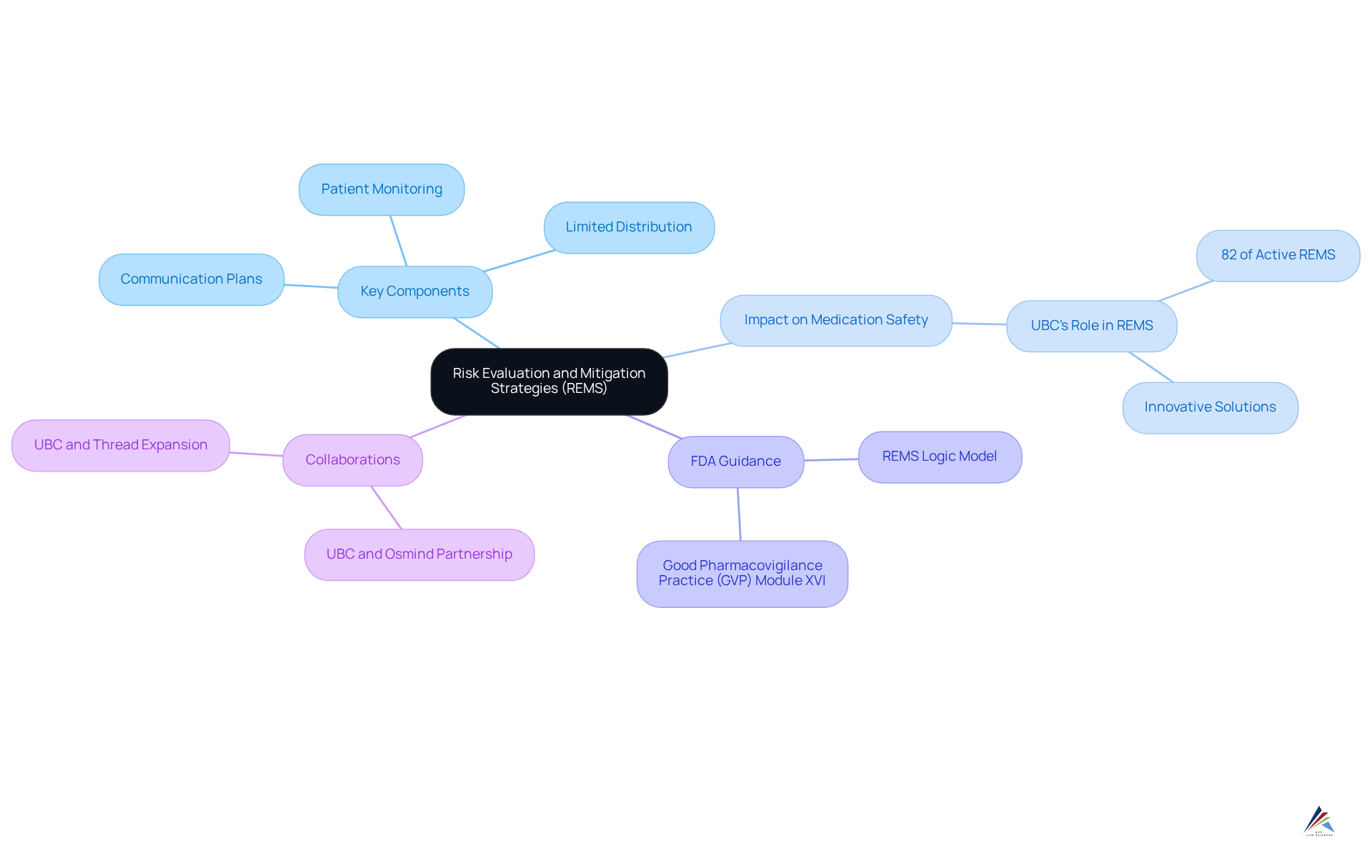
Risk Evaluation and Mitigation Strategies are essential frameworks established by the FDA to ensure that the benefits of certain medications outweigh their risks, particularly for drugs with significant safety concerns. These initiatives are pivotal in preventing adverse events by requiring pharmaceutical companies to actively monitor the use of these medications, thereby ensuring they are prescribed and dispensed safely. Key components of risk evaluation and mitigation strategies include:
- Communication plans
- Limited distribution
- Patient monitoring
All of which are critical for ensuring compliance within the pharmaceutical sector.
The impact of Risk Evaluation and Mitigation Strategies programs on medication safety is underscored by UBC's involvement in the design, development, or delivery of 82% of active strategies in the market. This extensive experience highlights the effectiveness of these strategies in managing medication-related risks. Additionally, UBC has been recognized by Everest Group as a leader in its 2025 Pre-approval Pharmacovigilance (PV) PEAK Matrix®, reinforcing its commitment to delivering compliant and patient-focused solutions.
Moreover, the FDA's recent draft guidance titled 'Logic Model: A Framework to Link Program Design With Assessment,' released on May 7, 2024, emphasizes the structured approach necessary for successful program implementation, focusing on the design, implementation, and evaluation phases. This guidance aligns with the emphasis on effective communication and oversight within these initiatives.
By ensuring that patients are well-informed about the risks associated with their medications, risk evaluation and mitigation strategies not only enhance patient safety but also contribute to the overall integrity of the drug approval process. The significance of these programs in cannot be overstated, as they serve as a crucial line of defense in protecting public health. Furthermore, collaborations such as that between UBC and Osmind aim to improve mental health treatment within this framework, showcasing innovative methods to enhance safe medication use and health outcomes. The revised GVP Module XVI, launched on August 6, 2024, also provides prescriptive guidance on risk reduction, underscoring the evolving nature of risk evaluation and its impact on medication safety compliance.
Identify REMS Program Drugs and Their Regulatory Context
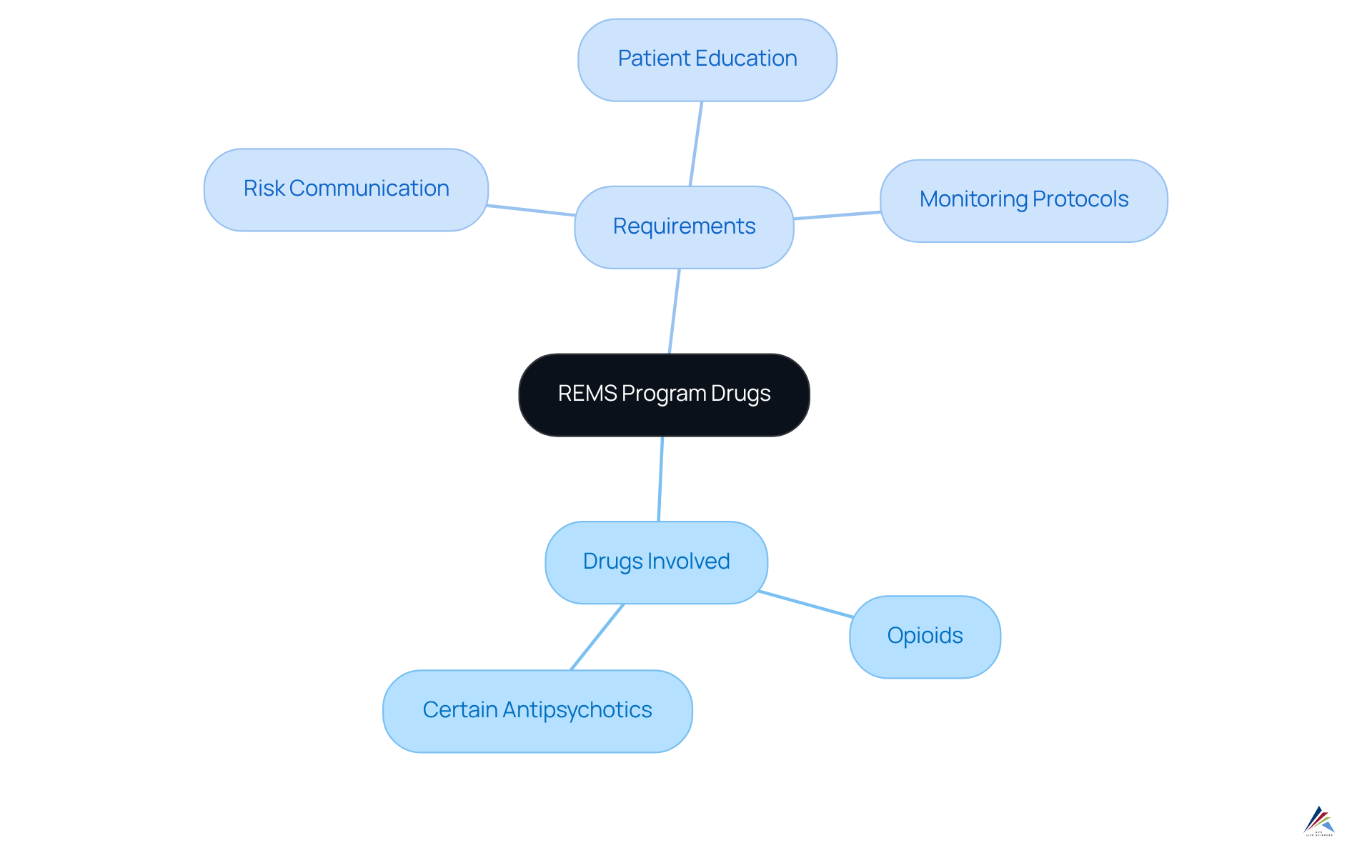
To effectively manage compliance with risk evaluation and mitigation strategies, it is essential to identify the medications involved in these initiatives. The FDA maintains a comprehensive list of REMS program drugs that are subject to risk evaluation and mitigation strategies, which includes treatments that pose significant risks, such as:
- Opioids
- Certain antipsychotics
Each risk evaluation and mitigation strategy entails specific requirements that must be strictly followed, encompassing:
- Risk communication
- Patient education
- Monitoring protocols
Understanding the surrounding these medications, including the FDA's role and the ramifications of non-compliance, is critical for pharmaceutical professionals tasked with overseeing risk evaluation and mitigation strategies.
Implement Compliance Strategies for REMS Program Drugs
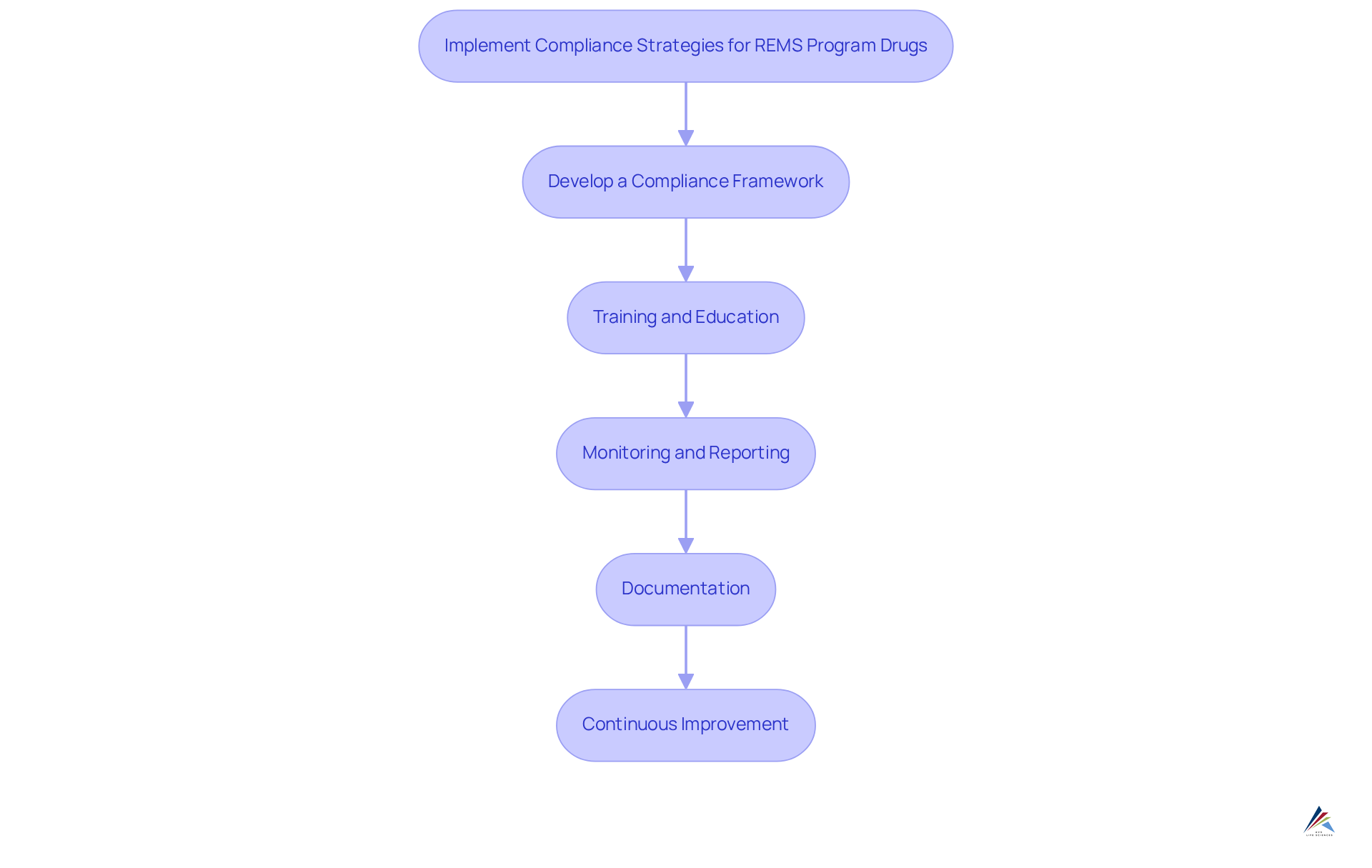
Implementing compliance strategies for REMS program drugs requires a systematic approach that addresses key challenges.
- Develop a Compliance Framework: Establish a clear structure that outlines the roles and responsibilities of all participants involved in the REMS program drugs and the Risk Evaluation and Mitigation Strategy. This framework serves as the foundation for effective compliance.
- : Conduct regular training sessions for healthcare providers and pharmacists to ensure they comprehend the requirements and their significance. Such education fosters a culture of compliance and awareness.
- Monitoring and Reporting: Establish robust systems for tracking adherence to REMS program drugs requirements, including monitoring patient outcomes and adverse events. This proactive approach enables timely interventions and enhances patient safety.
- Documentation: Maintain thorough records of all activities related to REMS program drugs, including training logs, patient communications, and audit reviews. Comprehensive documentation is crucial for accountability and regulatory compliance.
- Continuous Improvement: Regularly review and update adherence strategies based on feedback and changes in regulatory requirements. This commitment to improvement ensures that compliance efforts remain relevant and effective.
By implementing these strategies, organizations can navigate the complexities of drug distribution while safeguarding patient health.
Address Challenges in Managing REMS Compliance
Managing REMS compliance presents several significant challenges that organizations must navigate effectively:
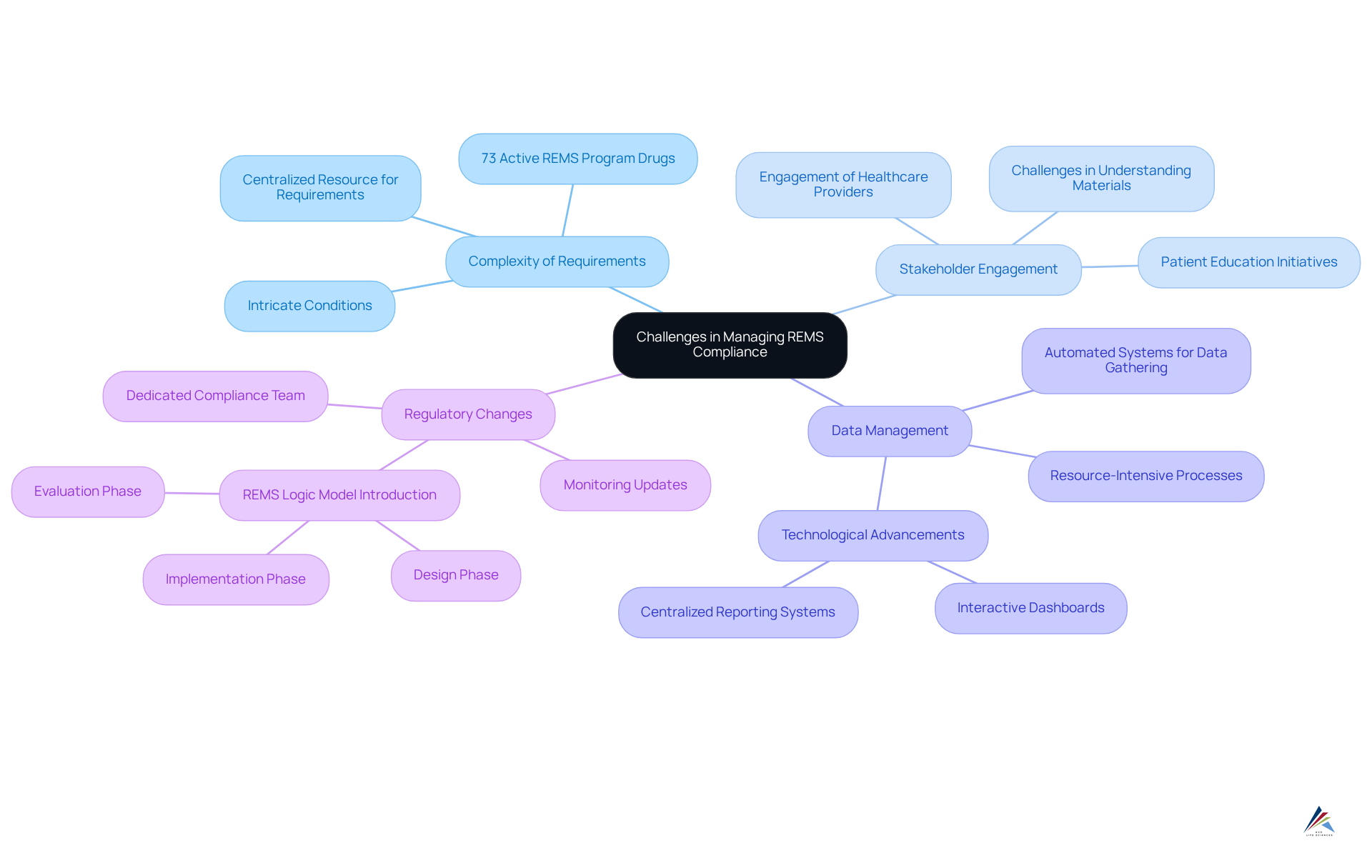
- Complexity of Requirements: Risk Evaluation and Mitigation Strategies often feature intricate conditions that vary by medication, complicating compliance efforts. As of January 2025, there are 73 active REMS program drugs as part of FDA risk evaluation and mitigation strategies, highlighting the extensive landscape organizations must navigate. To tackle this complexity, organizations should create a centralized resource that clearly details the specific requirements for each medication, ensuring that all team members have access to the necessary information.
- Stakeholder Engagement: Engaging all stakeholders—including healthcare providers, patients, and pharmacies—can be particularly challenging. Effective strategies involve consistent communication and educational initiatives that promote collaboration and guarantee that all parties comprehend their roles in the process. A qualitative study shows that while patients feel reassured about medication safety due to the REMS program drugs, many struggle with understanding educational materials and accessing treatments, highlighting the need for enhanced communication. As noted by A. Sarpatwari, "Participants reported feeling reassured about drug safety due to the REMS program drugs; however, many also expressed difficulties in understanding educational materials and obtaining the medications associated with these risk evaluation and mitigation strategies."
- Data Management: The gathering and examination of information related to risk evaluation and mitigation strategies can be resource-intensive. Introducing automated systems for data gathering and reporting can simplify this procedure, enabling organizations to concentrate on regulations instead of administrative burdens. Technological advancements, such as interactive dashboards and centralized reporting systems, are being developed to enhance the implementation of REMS program drugs by the FDA.
- Regulatory Changes: Staying informed about regulatory changes can be daunting for oversight teams. Establishing a dedicated compliance team to monitor updates and adjust strategies accordingly is essential. The FDA's recent introduction of the Logic Model aims to and foster collaboration among stakeholders, which can assist in adapting to regulatory shifts. This model offers a structured framework for designing, implementing, and assessing risk evaluation and mitigation strategies, emphasizing the significance of remaining updated on regulatory changes.
By proactively addressing these challenges, organizations can significantly enhance their efforts regarding REMS program drugs compliance, ultimately ensuring patient safety and improving access to necessary medications.
Conclusion
Mastering compliance with Risk Evaluation and Mitigation Strategies (REMS) is essential for ensuring the safe use of medications that carry significant risks. Recognizing the importance of these programs enables pharmaceutical professionals to navigate the complexities associated with REMS effectively, ultimately safeguarding patient health and enhancing the integrity of the drug approval process.
This article highlights several key strategies for successful REMS program compliance:
- Establishing a comprehensive compliance framework
- Providing ongoing training for healthcare providers
- Implementing robust monitoring systems
- Maintaining meticulous documentation
- Committing to continuous improvement
It also addresses the challenges organizations face in managing compliance, including:
- The complexity of requirements
- Stakeholder engagement
- Data management
- Staying informed about regulatory changes
As the landscape of REMS continues to evolve, it is imperative for stakeholders to remain proactive and informed. By fostering collaboration, enhancing communication, and leveraging technological advancements, organizations can overcome obstacles and ensure that the benefits of medications truly outweigh their risks. Emphasizing the significance of these strategies not only contributes to patient safety but also reinforces the critical role of REMS programs in the pharmaceutical industry.
Frequently Asked Questions
What are REMS programs?
REMS (Risk Evaluation and Mitigation Strategies) programs are frameworks established by the FDA to ensure that the benefits of certain medications outweigh their risks, particularly for drugs with significant safety concerns.
Why are REMS programs important?
REMS programs are important because they help prevent adverse events by requiring pharmaceutical companies to actively monitor the use of medications, ensuring they are prescribed and dispensed safely.
What are the key components of REMS programs?
The key components of REMS programs include communication plans, limited distribution, and patient monitoring.
How does UBC contribute to REMS programs?
UBC is involved in the design, development, or delivery of 82% of active REMS strategies in the market, highlighting their effectiveness in managing medication-related risks.
What recognition has UBC received for its work in pharmacovigilance?
UBC has been recognized by Everest Group as a leader in its 2025 Pre-approval Pharmacovigilance (PV) PEAK Matrix®, showcasing its commitment to compliant and patient-focused solutions.
What recent guidance has the FDA released regarding REMS programs?
The FDA released a draft guidance titled 'Logic Model: A Framework to Link Program Design With Assessment' on May 7, 2024, emphasizing the structured approach necessary for successful program implementation.
How do REMS programs enhance patient safety?
REMS programs enhance patient safety by ensuring that patients are well-informed about the risks associated with their medications, thus contributing to the overall integrity of the drug approval process.
What is the significance of collaborations in REMS programs?
Collaborations, such as that between UBC and Osmind, aim to improve mental health treatment within the REMS framework, showcasing innovative methods to enhance safe medication use and health outcomes.
What does the revised GVP Module XVI provide?
Launched on August 6, 2024, the revised GVP Module XVI provides prescriptive guidance on risk reduction, highlighting the evolving nature of risk evaluation and its impact on medication safety compliance.
