Master QMSR Compliance: Essential Steps for Pharmaceutical Officers

Overview
The article outlines the critical steps that pharmaceutical officers must undertake to achieve mastery over compliance with the Quality Management System Regulation (QMSR) within the medical device industry. It underscores the significance of:
- Risk management
- Thorough documentation
- A commitment to continuous improvement
as fundamental elements of QMSR. These components are further enhanced by aligning with ISO 13485, which not only bolsters patient safety but also streamlines compliance processes. This structured approach not only addresses compliance challenges but also offers detailed solutions, ultimately guiding the audience towards actionable insights and engagement with AVS Life Sciences.
Introduction
The Quality Management System Regulation (QMSR) represents a transformative approach to ensuring the safety and efficacy of medical devices, compelling pharmaceutical officers to adeptly navigate a complex landscape of compliance. This article explores the essential steps for mastering QMSR compliance, providing insights into the critical components that safeguard patient safety and streamline regulatory processes. As the industry adapts to these evolving standards, manufacturers must consider:
- How can they effectively prepare for the heightened scrutiny and operational demands that accompany this regulatory shift?
Define QMSR and Its Importance in Medical Device Compliance
The Quality Management System Regulation represents a pivotal shift in the FDA's approach to ensuring that medical devices are consistently manufactured to meet stringent quality standards. This regulation replaces the former Quality System Regulation (QSR) and closely aligns with international standards, particularly . The comprehensive framework of the quality management system addresses all aspects of the product lifecycle, from design and development to production and post-market surveillance. For pharmaceutical officers, adherence to QMSR regulations is not merely a regulatory obligation; it is essential for safeguarding patient safety and avoiding potential penalties.
Key components of the QMSR include:
- Risk Management: A robust emphasis on a risk-based approach throughout the product lifecycle, ensuring that potential hazards are effectively identified and mitigated.
- Documentation: Mandates stringent documentation practices, including the development of Standard Operating Procedures (SOPs), that bolster compliance and enhance traceability—critical for audits and regulatory reviews. This aligns with AVS Life Sciences' commitment to exemplary documentation practices.
- Continuous Improvement: Fosters a culture of ongoing evaluation and enhancement of management processes, promoting innovation and operational excellence, which are core principles of AVS Life Sciences' consulting services.
The transition to this new system is particularly significant given that annual fatalities linked to inadequate healthcare standards and safety in the U.S. are estimated at 98,000. Moreover, the WHO estimates that 134 million adverse events occur in hospitals each year in low- and middle-income countries, resulting in approximately 2.6 million deaths. The FDA's alignment with ISO 13485 aims to alleviate regulatory burdens on medical device manufacturers, which is expected to elevate the overall quality of medical devices available to consumers, thereby enhancing patient safety. As the healthcare landscape evolves, pharmaceutical executives must prioritize understanding and implementing management system regulations, including GXP and data integrity principles, to ensure compliance and uphold the highest standards in their operations—reflecting the proven excellence in life sciences consulting that AVS Life Sciences offers.
Explore the Harmonization of QMSR with ISO 13485 Standards
The alignment of the Quality Management System Regulation with ISO 13485 represents a pivotal shift in the regulatory landscape for medical device manufacturers. Recognized globally, ISO 13485 outlines the requirements for a management system that enables organizations to consistently deliver medical devices and services that meet customer and regulatory expectations.
Key benefits of this harmonization include:
- Streamlined Compliance: Aligning QMSR with ISO 13485 simplifies the compliance process for manufacturers, especially those operating across multiple jurisdictions. This alignment reduces the complexity of navigating various , allowing for a more unified management approach.
- Enhanced Assurance of Standards: The integration of ISO 13485 principles into the management system review cultivates a more robust framework. This integration underscores the importance of risk management and continuous improvement, both essential for upholding high standards in product safety and efficacy.
- Global Market Access: Compliance with QMSR and ISO 13485 not only facilitates easier access to international markets but also bolsters credibility. Numerous countries recognize ISO 13485 as a hallmark of excellence, making it a vital component for manufacturers aiming to expand their global footprint.
Real-world examples underscore the effectiveness of ISO 13485 adherence in medical device manufacturing. For instance, AVS Life Sciences successfully aided a leading biotechnology firm in enhancing their GMP facility, highlighting the importance of assurance and regulatory compliance. This project not only met the client's specifications but also ensured that their manufacturing processes adhered to stringent regulatory standards, including GXP and FDA regulations, ultimately elevating product quality and safety.
During the upgrade, AVS Life Sciences employed comprehensive methodologies, including thorough gap analysis, development of standard operating procedures (SOPs), and rigorous internal auditing techniques. These processes ensured that the facility met all essential regulatory standards while simultaneously enhancing operational efficiencies.
Companies that have embraced this standard report improved operational efficiencies and a reduction in compliance-related challenges. Manufacturers leveraging digital solutions to comply with ISO 13485 have experienced significant decreases in errors and improved traceability, both crucial for satisfying regulatory demands.
The benefits of aligning the quality management system with ISO 13485 extend beyond mere compliance; they encompass improved product performance and safety, ultimately fostering greater customer trust and satisfaction. As the FDA continues to update its regulations, the alignment of these standards is expected to enhance adherence rates throughout the industry, equipping manufacturers for success in a competitive global market.
Prepare for QMSR Compliance: Key Steps for Manufacturers
Preparing for compliance with quality management system requirements necessitates a series of critical steps that producers must undertake to align their quality management systems with the evolving regulations. Consider the following key actions:
- Conduct a Gap Analysis: A thorough Quality System Assessment is vital for evaluating current practices against the qmsr requirements, pinpointing areas that require enhancement. This proactive approach aids producers in circumventing potential non-compliance issues as the February 2026 deadline approaches. AVS Life Sciences provides expert assessments to navigate this process effectively.
- Update Documentation: It is imperative to to meet standards. This includes the creation of a Medical Device File that consolidates records previously included in the Device Master Record, ensuring that all processes are meticulously documented and readily accessible for FDA inspections. AVS Life Sciences can assist in the development and updating of these essential documents to align with regulatory expectations.
- Train Staff: Implementing robust training programs is crucial for ensuring that employees comprehend quality management system regulations and their respective roles in maintaining compliance. Statistics reveal that 40% of organizations face significant challenges in training employees, underscoring the necessity for effective training initiatives. AVS Life Sciences offers tailored training solutions to enhance staff proficiency in regulatory matters.
- Implementing risk management practices by adopting ISO 14971 aligns with the expectations of qmsr, emphasizing the identification and mitigation of risks throughout the product lifecycle. This approach not only strengthens compliance but also elevates overall product quality. AVS Life Sciences specializes in risk management strategies that assist producers in navigating these complexities.
- Establishing internal audits: Developing a schedule for regular internal audits is essential for assessing adherence to the QMSR and identifying areas for continuous improvement. Conducting simulated FDA inspections can also prepare producers effectively for actual inspections, ensuring readiness and compliance with the new regulations. AVS Life Sciences offers comprehensive audit services to support producers in this critical area.
By implementing these measures, producers can position themselves for success in managing the complexities of compliance, ultimately enhancing their management practices and operational efficiency.

Understand Changes in FDA Inspection Processes Under QMSR
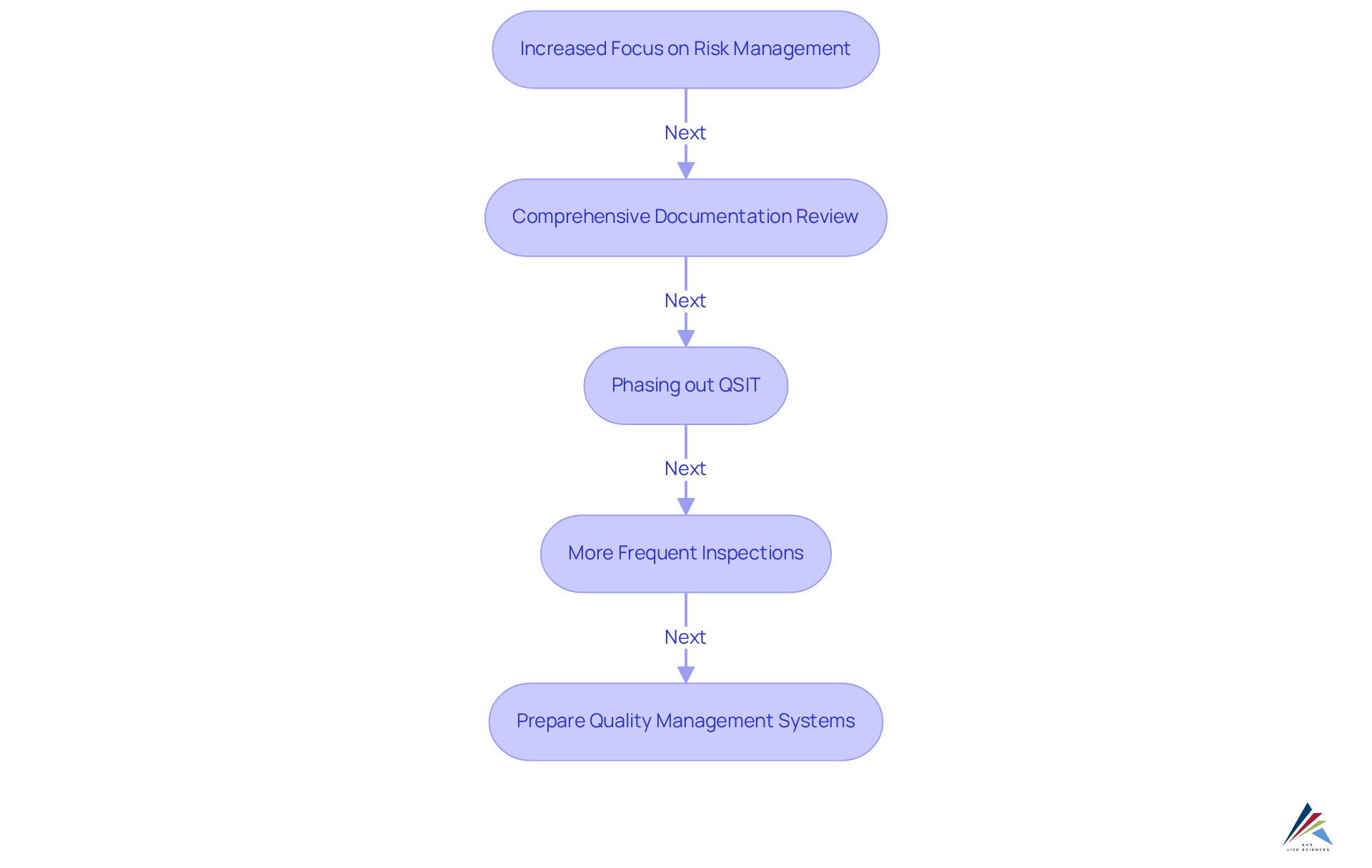
The transition to the qmsr will significantly transform the FDA's inspection procedures for medical device manufacturers. Key changes include:
- Increased Focus on Risk Management: Inspections will emphasize the effectiveness of risk management practices. Manufacturers must clearly demonstrate their methodologies for identifying and mitigating risks, aligning with the FDA's objective of enhancing device safety and efficacy.
- Comprehensive Documentation Review: FDA inspectors will conduct thorough reviews of a broader range of documentation, including management reviews, internal audits, and supplier audit records. This comprehensive approach ensures that manufacturers are fully compliant with regulatory requirements, as the FDA intends to scrutinize all aspects of quality management systems.
- Phasing out QSIT: The Quality System Inspection Technique (QSIT) will be phased out and replaced by a new inspection methodology that aligns with the requirements of qmsr. This transition reflects the FDA's commitment to harmonizing its inspection processes with international standards, particularly ISO 13485.
- More Frequent Inspections: Manufacturers may face more frequent inspections as the FDA intensifies its oversight to ensure compliance with the new regulations. This increased scrutiny aims to foster a culture of excellence and accountability within the industry.
To effectively prepare for these changes, manufacturers should proactively . This entails ensuring that all documentation is current, comprehensive, and readily available for inspection, thereby facilitating a smoother transition to the new regulatory landscape.
Conclusion
The Quality Management System Regulation (QMSR) serves as a pivotal framework that guarantees the manufacturing of medical devices to high-quality standards, thus safeguarding patient safety and ensuring compliance with regulatory mandates. For pharmaceutical officers, grasping and implementing QMSR transcends mere regulatory adherence; it embodies the cultivation of a quality-centric culture that ultimately bolsters the safety and efficacy of medical devices available in the market.
This article has underscored the significance of QMSR, particularly its alignment with ISO 13485, which not only streamlines compliance but also enhances operational efficiencies. Manufacturers can achieve compliance by undertaking key steps such as:
- Conducting gap analyses
- Updating documentation
- Training personnel
- Implementing risk management practices
- Establishing internal audits
Each of these actions is crucial in preparing for regulatory changes and ensuring that manufacturers are well-equipped to meet the FDA's evolving expectations.
As the regulatory landscape for medical devices continues to transform, it is imperative for manufacturers to prioritize QMSR compliance. By adopting these essential steps and fostering a proactive approach to quality management, organizations can effectively navigate the complexities of compliance while contributing to improved patient outcomes and safety. The commitment to quality transcends regulatory obligation; it is a fundamental element in establishing trust and credibility within the pharmaceutical industry.
Frequently Asked Questions
What is QMSR and why is it important in medical device compliance?
The Quality Management System Regulation (QMSR) is a regulation by the FDA that ensures medical devices are consistently manufactured to meet strict quality standards. It replaces the former Quality System Regulation (QSR) and aligns closely with international standards like ISO 13485. QMSR is crucial for safeguarding patient safety and avoiding potential penalties.
What are the key components of QMSR?
The key components of QMSR include Risk Management, which emphasizes a risk-based approach throughout the product lifecycle; Documentation, which mandates stringent documentation practices such as developing Standard Operating Procedures (SOPs); and Continuous Improvement, which promotes ongoing evaluation and enhancement of management processes.
How does QMSR relate to patient safety?
QMSR is designed to enhance patient safety by ensuring that potential hazards are effectively identified and mitigated throughout the product lifecycle. The regulation aims to elevate the overall quality of medical devices, thereby reducing the risk of adverse events linked to inadequate healthcare standards.
What is the significance of aligning QMSR with ISO 13485?
The alignment of QMSR with ISO 13485 is significant because it aims to alleviate regulatory burdens on medical device manufacturers while enhancing the overall quality of medical devices. This alignment helps ensure compliance with international standards, ultimately benefiting patient safety.
Why should pharmaceutical executives prioritize understanding QMSR?
Pharmaceutical executives should prioritize understanding QMSR to ensure compliance with management system regulations and uphold high standards in their operations. This understanding is essential for safeguarding patient safety and reflects the proven excellence in life sciences consulting that organizations like AVS Life Sciences offer.
