Master MoCRA Compliance: Essential Steps for Pharmaceutical Officers

Overview
Pharmaceutical officers must master essential steps for MoCRA compliance, which include:
- Registering facilities
- Listing products
- Adhering to Good Manufacturing Practices (GMP)
- Maintaining thorough documentation
These requirements are crucial for ensuring consumer safety and accountability. Furthermore, collaboration with consulting firms like AVS Life Sciences is vital for achieving effective compliance and maintaining operational integrity in the ever-evolving regulatory landscape. By engaging with AVS Life Sciences, pharmaceutical officers can navigate compliance challenges more effectively, ensuring they meet both regulatory demands and consumer expectations.
Introduction
The Modernization of Cosmetics Regulation Act (MoCRA) signifies a pivotal transformation in the regulation of cosmetic products in the United States, enhancing the FDA's authority and placing a premium on consumer safety. In this evolving landscape, pharmaceutical officers are tasked with the critical responsibility of ensuring compliance with stringent new requirements that are set to reshape the industry. With the complexities of facility registration, product listing, and Good Manufacturing Practices at the forefront, how can these professionals effectively adapt to uphold both compliance and operational integrity? This question underscores the urgency for tailored compliance solutions that not only meet regulatory demands but also fortify the industry's commitment to safety and quality.
Understand the MoCRA: Overview and Importance
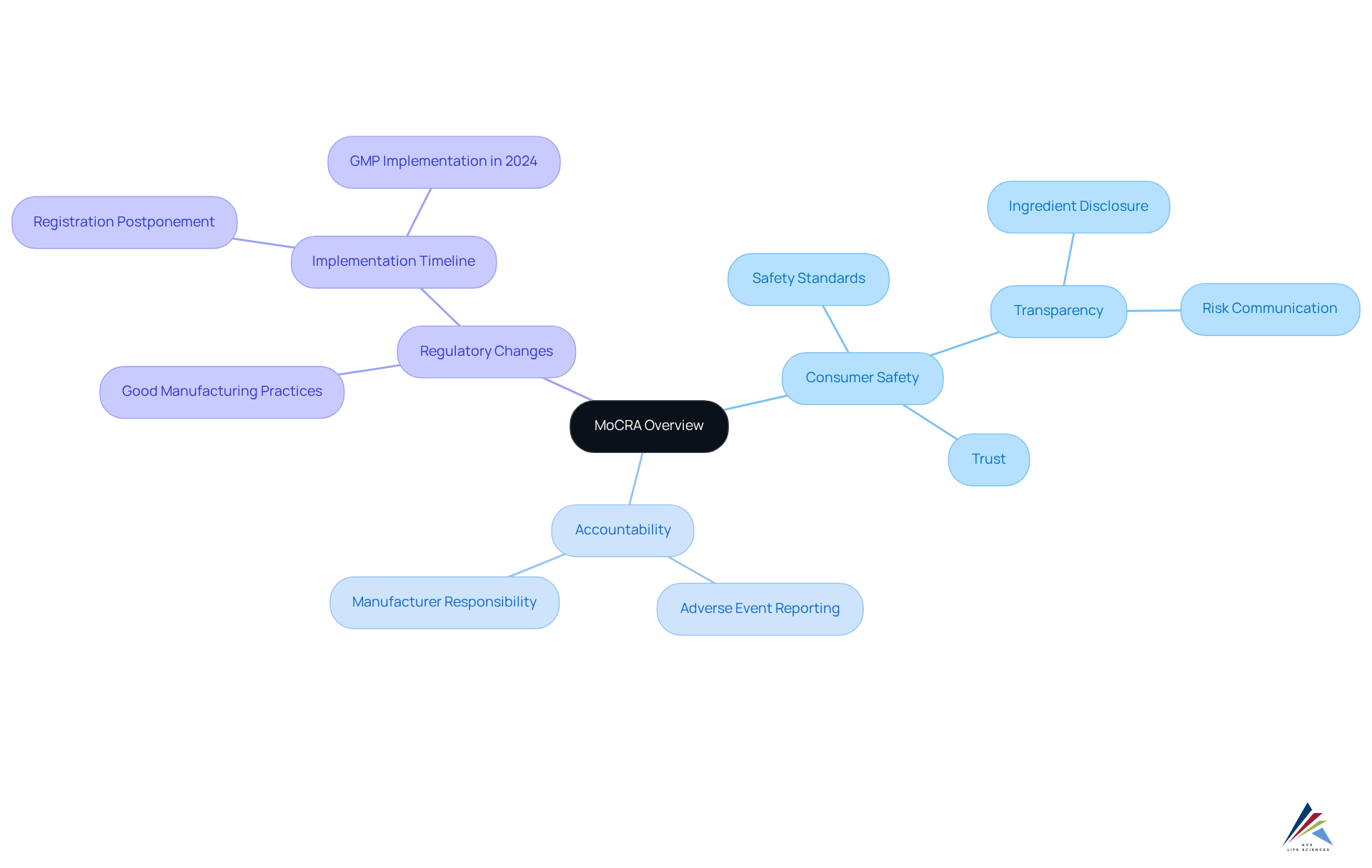
The MoCRA (Modernization of Cosmetics Regulation Act), enacted in December 2022, signifies a pivotal transformation in the regulatory landscape for cosmetics in the United States. This legislation greatly strengthens the FDA's power to regulate cosmetic items, emphasizing the assurance of their effectiveness and security. The key objectives of MoCRA include:
- Consumer Safety: The Act establishes rigorous safety standards designed to protect consumers from harmful ingredients and unsafe products. This dedication to security is highlighted by the necessity for manufacturers to perform comprehensive assessments of their items prior to market introduction. The MoCRA requires that manufacturers offer comprehensive disclosures concerning item ingredients and potential risks to ensure transparency. This transparency fosters trust between consumers and brands, empowering individuals to .
- Accountability: Companies are now held accountable for the safety of their products, with stringent requirements for reporting adverse events. Manufacturers must promptly report serious adverse reactions, enabling regulatory bodies to swiftly address potential risks.
As the FDA prepares to implement these regulations, including mandatory Good Manufacturing Practices (GMP) starting in 2024, companies are adapting to ensure compliance. For example, numerous companies are collaborating with external fulfillment providers to manage the intricacies of regulatory compliance, improving operational efficiency and risk reduction. The revised regulations not only seek to enhance consumer protection but also to establish a more transparent supply chain, enabling quicker responses to concerns.
The significance of consumer protection in MoCRA cannot be overstated. As noted by FDA officials, the MoCRA significantly expands the agency's capacity to oversee and ensure the safety of cosmetic products, a change that is unparalleled since the Federal Food, Drug, and Cosmetic Act of 1938. This evolution in regulation is crucial for maintaining high standards in the cosmetics industry and protecting public health. Moreover, AVS Life Sciences provides extensive quality management and regulatory adherence solutions that can help companies in managing these new requirements efficiently. By utilizing AVS Life Sciences' proficiency, companies can guarantee they adhere to regulatory standards while improving their operational practices. Moreover, the delay in the implementation of facility registration and item listing requirements until July 1, 2024, gives companies extra time to adhere to these new regulations.
Identify Key Compliance Requirements Under MoCRA
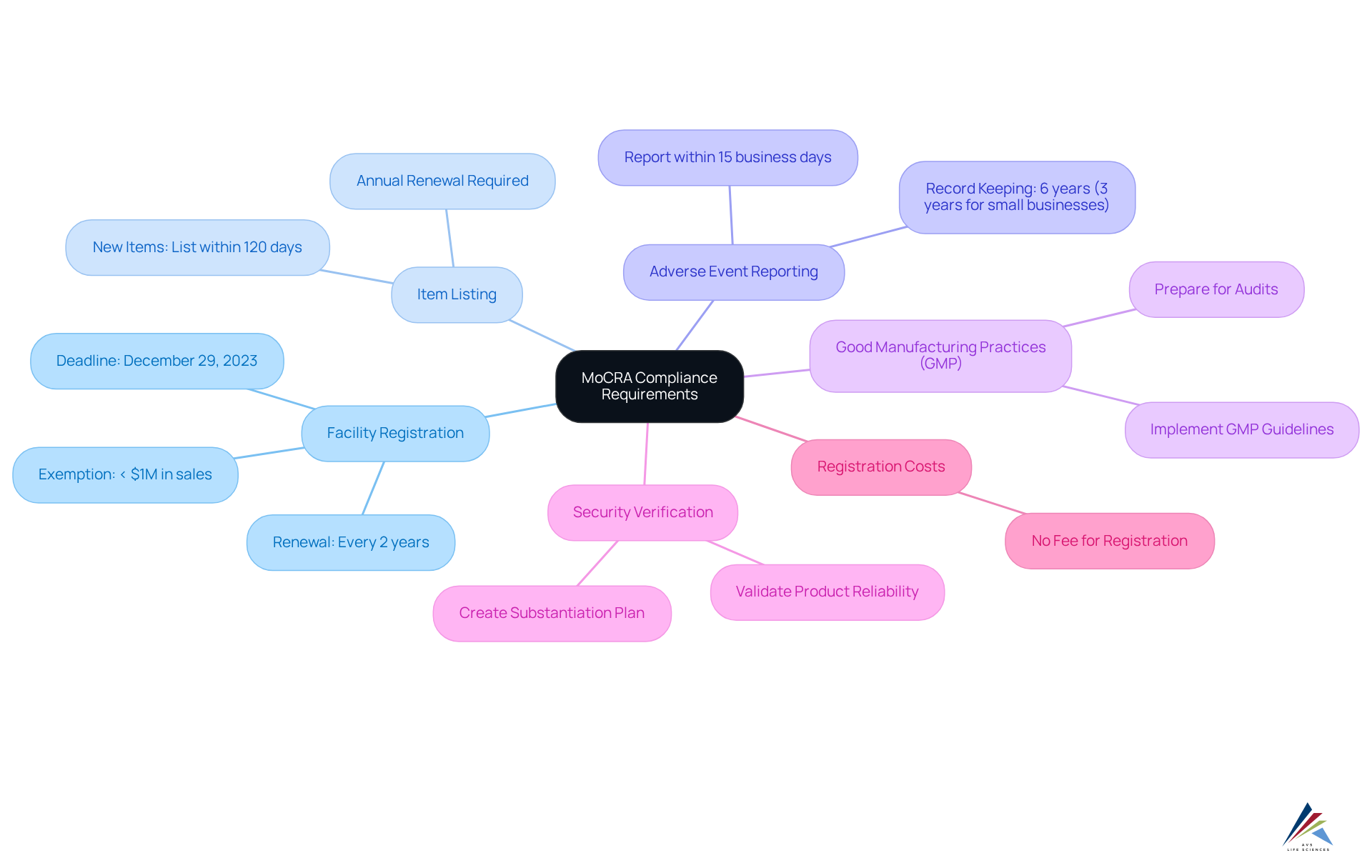
To ensure compliance with the Modernization of Cosmetics Regulation Act (MoCRA), pharmaceutical officers must focus on several essential requirements, leveraging the expertise of AVS Life Sciences for tailored consulting solutions in quality management and regulatory compliance:
- Facility Registration: All cosmetic manufacturing facilities are mandated to register with the FDA by December 29, 2023, and renew their registration every two years. This process guarantees that the FDA maintains current information regarding the locations of cosmetic production, including the name of the owner/operator, facility name, physical address, email address, and telephone number.
- Item Listing: Companies must provide a thorough inventory of all cosmetic items they produce, outlining components and intended applications. New items must be listed within 120 days of marketing, while existing ones must be renewed each year. AVS Life Sciences can assist in creating effective listing strategies to ensure compliance.
- Adverse Event Reporting: A designated responsible individual must report serious adverse events linked to cosmetic items to the FDA within 15 business days. This includes maintaining records of each adverse event for six years, or three years for small businesses. AVS Life Sciences offers guidance on establishing robust reporting mechanisms.
- Good Manufacturing Practices (GMP): Adhering to GMP guidelines is essential for ensuring quality and security. Facilities must implement these practices to meet regulatory expectations and prepare for audits. AVS Life Sciences offers extensive quality management solutions to assist organizations in following GMP standards, including documentation practices and GXP adherence.
- Security Verification: Companies must validate the reliability of their products before marketing, ensuring that sufficient records support their claims of protection. This involves creating a substantiation plan to tackle any data gaps prior to the regulatory deadline. AVS Life Sciences can assist in creating effective risk substantiation strategies.
- Registration Costs: It is crucial to recognize that the FDA will not impose a charge for facility registration under the new regulations, which may affect adherence strategies for pharmaceutical officials.
- Exemptions: Facilities with average gross annual sales of less than $1 million are exempt from registration, which is a significant consideration for smaller businesses.
By thoroughly grasping these needs and interacting with AVS Life Sciences, pharmaceutical officers can develop effective adherence strategies that align with the MoCRA (Modernization of Cosmetics Regulation Act), thus improving their organization's regulatory status and item security.
Register Facilities and List Products Effectively
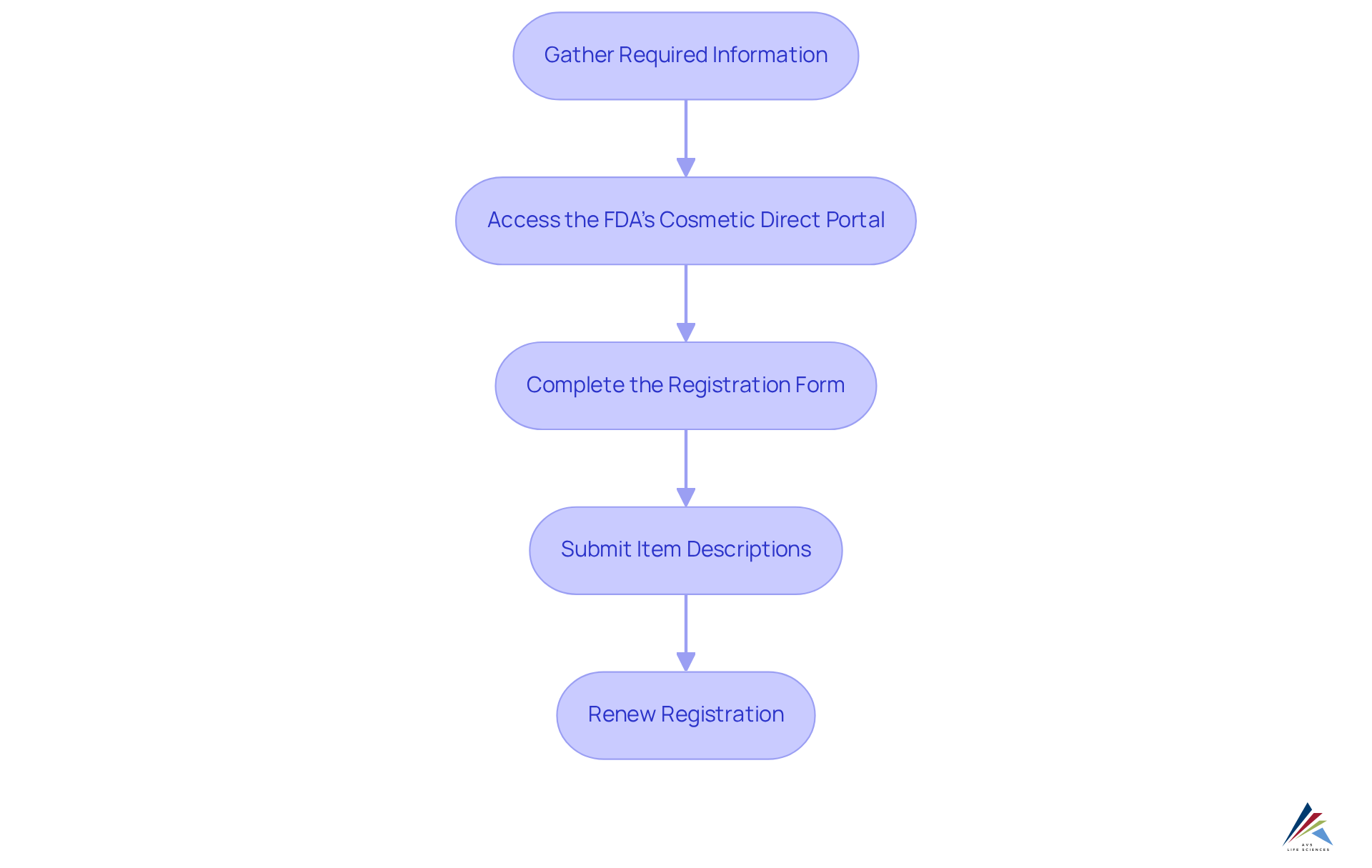
To effectively register facilities and list products under the Modernization of Cosmetics Regulation Act (MoCRA), it is imperative to follow these essential steps:
- Gather Required Information: Compile necessary details, including the facility name, address, contact information, and the names of responsible persons.
- Access the FDA's Cosmetic Direct Portal: Navigate to the FDA's Cosmetic Direct portal to commence the registration process.
- Complete the Registration Form: Accurately fill out the registration form, ensuring all information is current and complete to avoid common errors that could delay approval.
- Submit Item Descriptions: For each cosmetic item, provide comprehensive details, including ingredients, intended use, and safety data, to meet regulatory standards.
- Renew Registration: Remember to renew your facility registration every two years to uphold the necessary regulations.
By adhering to these steps, pharmaceutical officers can ensure their facilities are correctly registered and their products are listed in accordance with MoCRA (modernization of cosmetics regulation act). This diligence not only mitigates potential pitfalls but also reinforces regulatory adherence, fostering a compliant operational environment.
Implement Good Manufacturing Practices (GMP) for Compliance
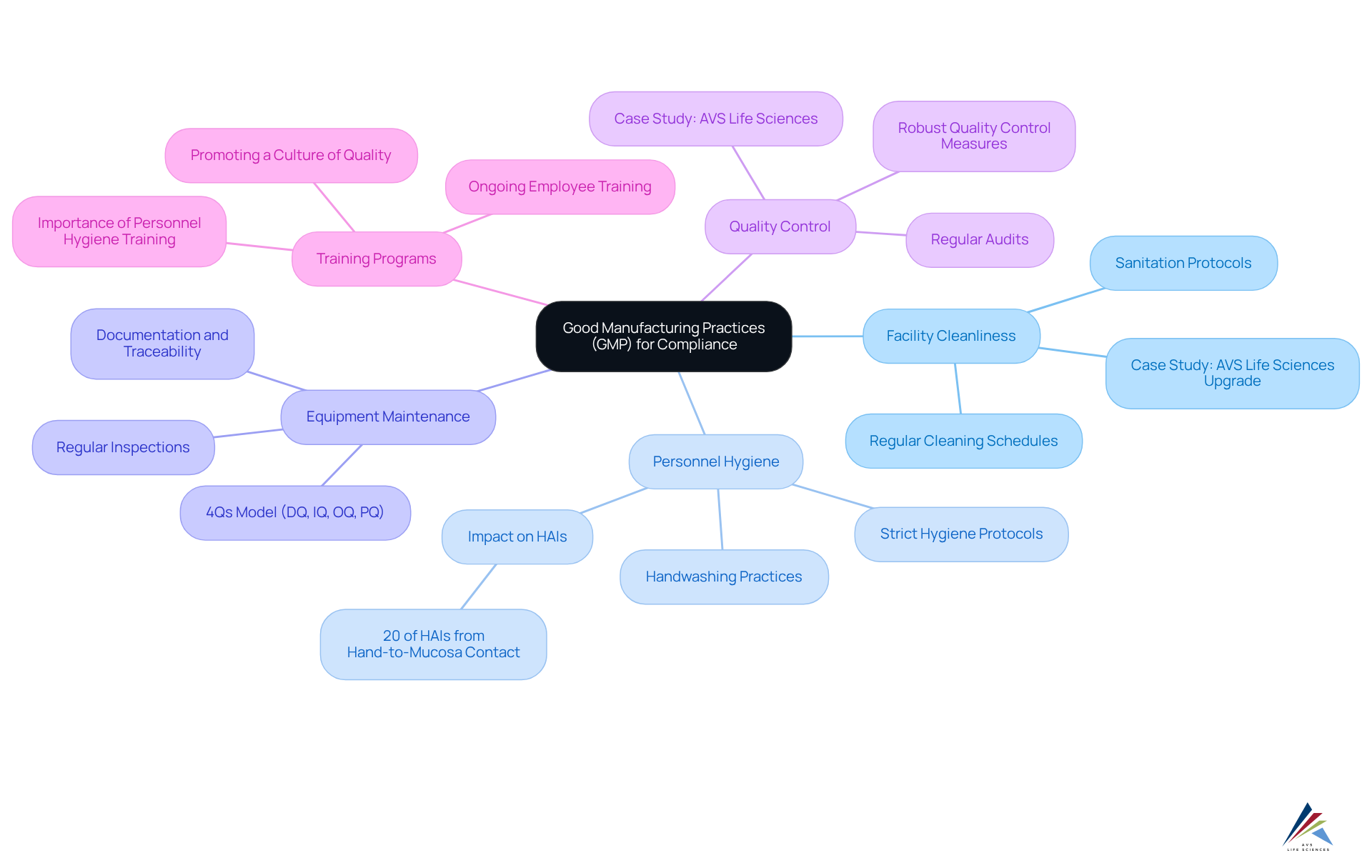
To effectively implement Good Manufacturing Practices (GMP) and ensure compliance with GXP and FDA regulations, pharmaceutical officers must concentrate on several key areas:
- Facility Cleanliness: A clean and sanitary environment is essential to prevent contamination. Regular cleaning schedules and adherence to sanitation protocols are vital for maintaining facility integrity. For instance, AVS Life Sciences successfully upgraded a biotechnology facility from a Biosafety Level 1 to a Level 2 GMP facility, demonstrating the importance of maintaining high cleanliness standards in compliance with regulatory requirements.
- Personnel Hygiene: Strict hygiene protocols must be enforced among all staff, including regular handwashing, appropriate attire, and the use of personal protective equipment (PPE). Effective personnel hygiene initiatives in cosmetics firms have shown considerable enhancements in safety. Research suggests that can significantly lower contamination risks. Notably, statistics indicate that 20% of healthcare-associated infections (HAIs) are related to hand-to-mucosa contact, underscoring the critical role of hand hygiene.
- Equipment Maintenance: Regular inspections and maintenance of equipment are crucial to ensure it operates correctly and safely. This includes adhering to the 4Qs model—Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ)—to validate equipment suitability and performance. AVS Life Sciences emphasizes the importance of thorough documentation and traceability during equipment upgrades to meet GMP standards.
- Quality Control: Establishing robust quality control measures is essential for monitoring production processes and ensuring product quality. This encompasses regular audits and adherence checks to align with GMP standards. The case study of AVS Life Sciences illustrates how effective quality control practices can lead to successful outcomes, such as identifying and addressing anomalies in test results during the upgrade process.
- Training Programs: Ongoing training for employees on GMP standards and practices is critical. Effective training programs not only improve adherence but also promote a culture of security and quality within the organization. As Anuradha Shenvi emphasizes, good personnel hygiene practices are foundational to safeguarding quality.
By focusing on these aspects, businesses can greatly improve their adherence to regulations, particularly the mocra (modernization of cosmetics regulation act), and guarantee the integrity and standard of their cosmetic items. As highlighted by GMP experts, ensuring facility cleanliness and personnel hygiene is essential for attaining high standards in product safety.
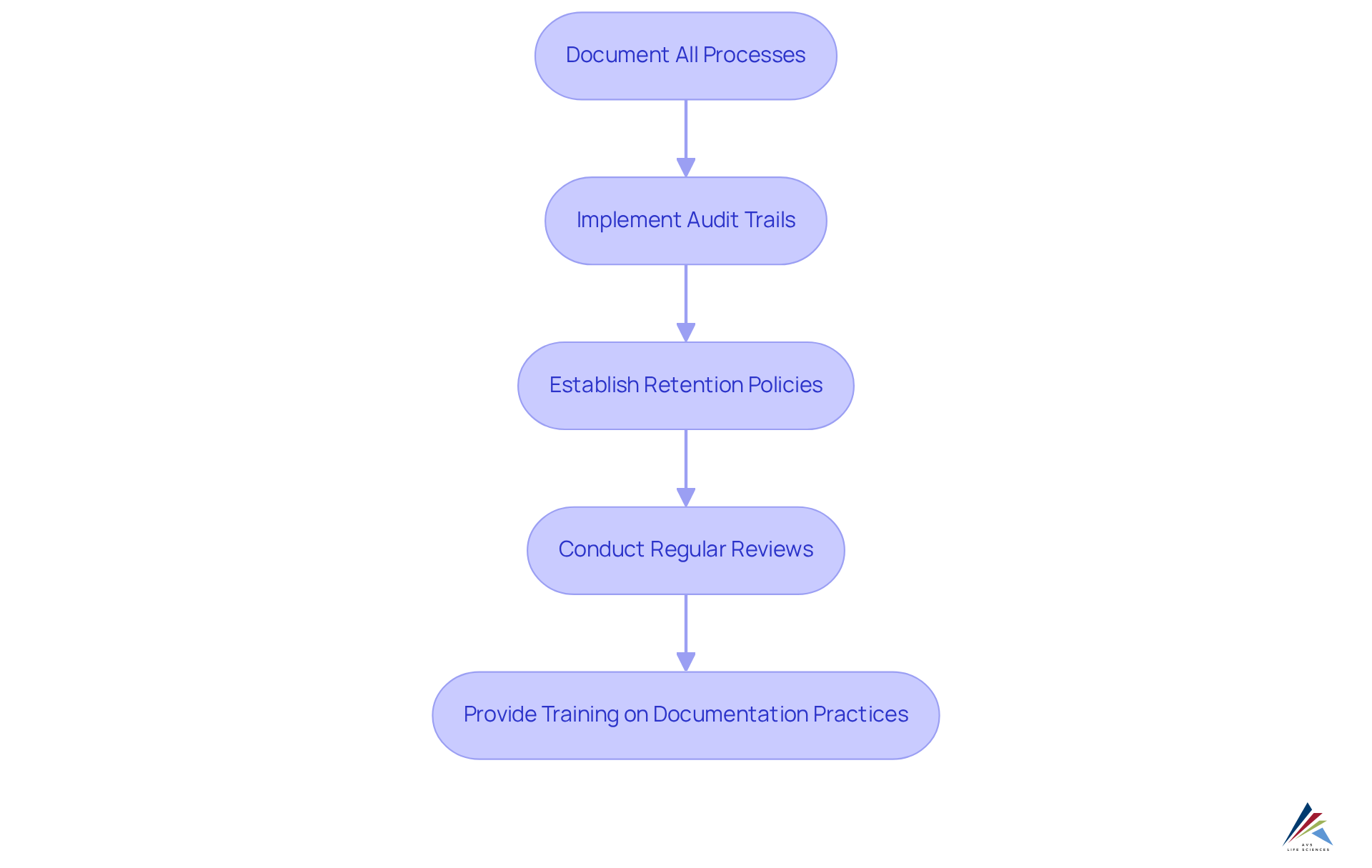
Maintain Documentation and Audit Trails for Compliance
To ensure effective documentation and audit trails for MoCRA compliance, it is imperative to consider the following guidelines:
- Document All Processes: Maintain comprehensive records of all manufacturing activities, including ingredient sourcing, production methods, and quality control measures.
- Implement Audit Trails: Utilize systems that track changes in documentation, ensuring every modification is logged with timestamps and user identification to enhance accountability.
- Establish Retention Policies: Define clear policies regarding the duration for which records must be retained, aligning with regulatory requirements to avoid potential adherence issues.
- Conduct Regular Reviews: Schedule periodic evaluations of documentation to verify accuracy and completeness, which is essential for upholding standards and preparing for audits.
- Provide Training on Documentation Practices: Educate staff on the importance of precise documentation and the methods for maintaining detailed records, fostering a culture of adherence within the organization.
By implementing these practices, pharmaceutical officers can effectively prepare their organizations for audits and inspections. This proactive approach not only strengthens compliance with MoCRA (modernization of cosmetics regulation act) but also enhances overall operational integrity.
Conclusion
The Modernization of Cosmetics Regulation Act (MoCRA) signifies a pivotal transformation in the regulatory framework governing cosmetic products within the United States. By augmenting the FDA's authority to oversee cosmetic safety, MoCRA is designed to fortify consumer protection through rigorous safety standards and accountability measures. It is essential for pharmaceutical officers to grasp and implement the key compliance requirements delineated by this legislation to ensure their organizations align with the new expectations.
This article outlines critical steps for compliance, including:
- Facility registration
- Item listing
- Adverse event reporting
- Adherence to Good Manufacturing Practices (GMP)
Each requirement is integral to safeguarding consumer health and upholding industry integrity. By leveraging resources such as AVS Life Sciences, companies can adeptly navigate the complexities of MoCRA compliance, fulfilling their obligations while enhancing operational efficiency.
Ultimately, MoCRA transcends being merely a regulatory hurdle; it presents an opportunity for companies to elevate their commitment to consumer safety and transparency. By embracing these new standards, organizations can cultivate trust with their customers and establish a benchmark for quality in the cosmetics industry. It is imperative for pharmaceutical officers to act decisively, implementing these compliance measures not only to meet regulatory requirements but also to contribute to a safer, more accountable cosmetics marketplace.
Frequently Asked Questions
What is the MoCRA and why is it important?
The MoCRA (Modernization of Cosmetics Regulation Act), enacted in December 2022, is a significant change in the U.S. cosmetics regulatory landscape. It enhances the FDA's authority to regulate cosmetic products, ensuring their safety and effectiveness, and establishes rigorous safety standards to protect consumers from harmful ingredients.
What are the main objectives of the MoCRA?
The main objectives of the MoCRA include ensuring consumer safety through comprehensive product assessments, holding companies accountable for product safety, improving transparency regarding ingredient disclosures, and establishing a more efficient regulatory framework.
What are the compliance requirements for cosmetic manufacturing facilities under MoCRA?
Compliance requirements include facility registration with the FDA by December 29, 2023, annual item listing of cosmetic products, adverse event reporting within 15 business days, adherence to Good Manufacturing Practices (GMP), and security verification of product claims.
When must cosmetic manufacturing facilities register with the FDA?
All cosmetic manufacturing facilities must register with the FDA by December 29, 2023, and renew their registration every two years.
What is required for item listing under MoCRA?
Companies must provide a detailed inventory of all cosmetic items, including components and intended applications. New items must be listed within 120 days of marketing, while existing items must be renewed annually.
What are the requirements for reporting adverse events?
A designated individual must report serious adverse events linked to cosmetic items to the FDA within 15 business days and maintain records of each adverse event for six years, or three years for small businesses.
What are Good Manufacturing Practices (GMP) and why are they important?
GMP are guidelines that ensure the quality and safety of cosmetic products. Facilities must implement these practices to meet regulatory expectations and prepare for audits, helping to enhance consumer safety.
Is there a cost associated with facility registration under MoCRA?
No, the FDA will not impose a charge for facility registration under the new regulations.
Are there any exemptions for small businesses under MoCRA?
Yes, facilities with average gross annual sales of less than $1 million are exempt from registration, which is significant for smaller businesses.
How can companies ensure compliance with MoCRA?
Companies can work with experts like AVS Life Sciences to develop tailored consulting solutions in quality management and regulatory compliance, ensuring they meet MoCRA requirements effectively.
