Master Life Sciences Compliance: Key Strategies for Success

Overview
Mastering life sciences compliance presents significant challenges, necessitating a comprehensive understanding of regulatory frameworks, the establishment of robust compliance programs, and the integration of technology for effective monitoring.
Organizations must navigate complex regulations such as GMP and ISO, define the responsibilities of compliance officers, and implement tailored third-party risk management strategies.
Leveraging technology to enhance monitoring is not merely beneficial; it is essential for maintaining operational integrity and avoiding severe penalties.
By addressing these critical areas, organizations can ensure compliance and foster a culture of accountability, ultimately safeguarding their operations and reputation.
Introduction
Navigating the intricate world of life sciences compliance presents significant challenges, as organizations face a labyrinth of regulations that vary widely across regions and product types. The stakes are high; non-compliance can lead to substantial fines and damaging product recalls. Therefore, it is crucial for companies to adopt effective strategies that ensure they remain ahead of the curve. This article explores essential practices that empower compliance officers and organizations to cultivate a culture of regulatory adherence. However, what transpires when the systems designed to ensure compliance become overwhelming or ineffective? Delving into this tension uncovers the pathway to mastering life sciences compliance.
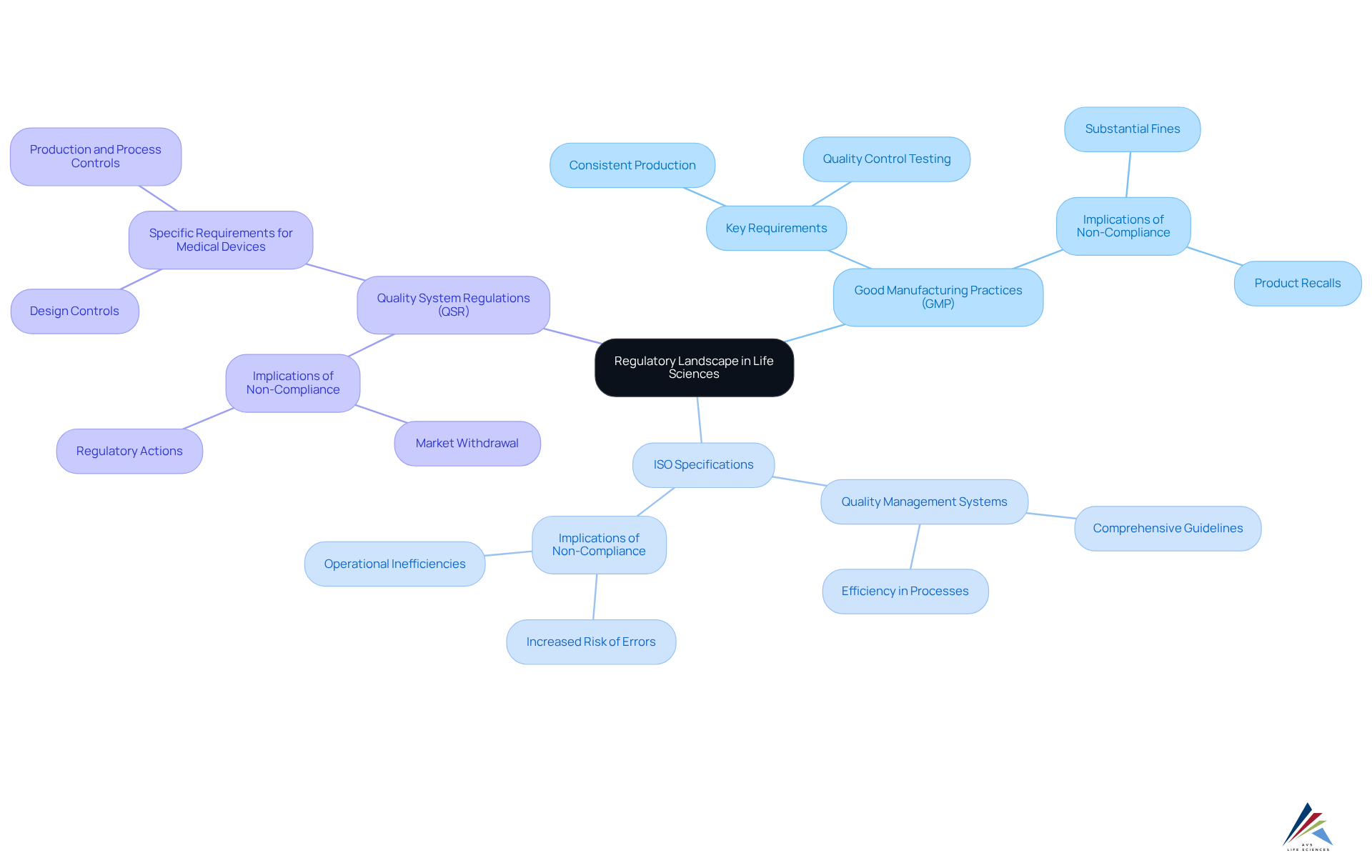
Understand the Regulatory Landscape in Life Sciences
The life sciences compliance within the industry requires navigating a complex framework of regulations that vary by region and product type. Central to this framework are Good Manufacturing Practices (GMP), which mandate that products are consistently produced and controlled according to stringent quality criteria. Additionally, ISO specifications offer comprehensive guidelines for developing efficient quality management systems, while Quality System Regulations (QSR) outline specific requirements for medical devices.
It is imperative to remain vigilant regarding life sciences compliance and any amendments to these regulations, as non-compliance can lead to severe consequences, including substantial fines and product recalls. To ensure adherence and align all operational processes with current criteria, for staff are not just beneficial, but essential.
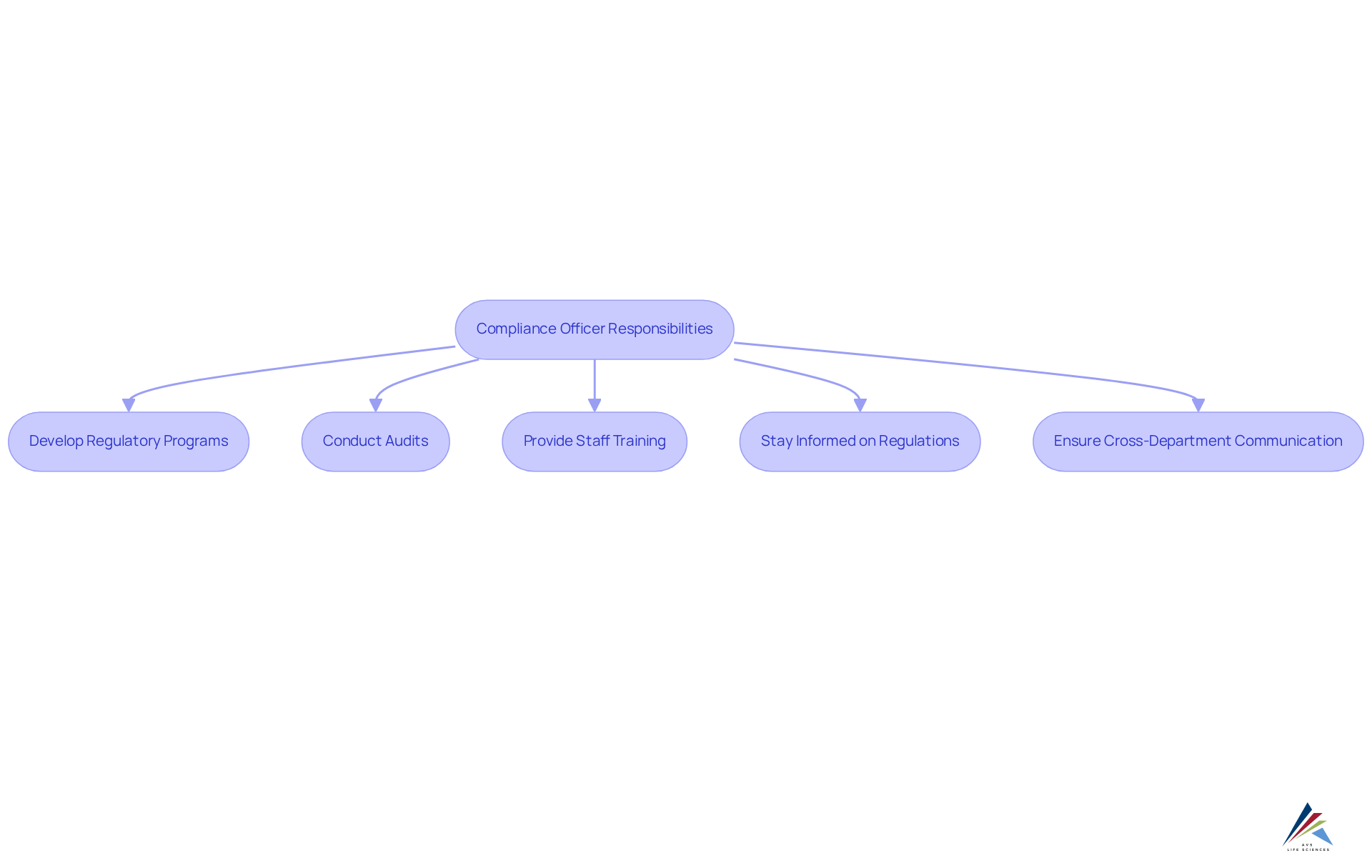
Define the Role and Responsibilities of Compliance Officers
Compliance officers play a pivotal role in ensuring that life sciences organizations adhere to life sciences compliance and uphold exemplary quality standards. Their responsibilities encompass the development and implementation of robust regulatory programs, conducting regular audits, and providing comprehensive training to staff on regulatory matters. Staying informed about regulatory changes is crucial, as these can profoundly influence organizational practices. Effective communication and collaboration across various departments are essential, enabling oversight officials to integrate regulations into every facet of the business.
A prime illustration of this is AVS Life Sciences' successful upgrade of a biotechnology GMP facility, wherein they facilitated a leading San Francisco-based biotech company’s transition from a Biosafety Level 1 to a Level 2 GMP facility. This initiative not only met regulatory standards but also enhanced quality assurance processes, underscoring the importance of life sciences compliance in maintaining operational integrity. Specific methodologies employed during this upgrade included comprehensive gap analysis and equipment installation, which led to identifying anomalies in test results and implementing corrective actions. By clearly delineating roles and responsibilities, organizations can cultivate a culture of compliance that mitigates risks and bolsters operational integrity.
This proactive approach is substantiated by the fact that nearly 90% of Chief Compliance Officers (CCOs) report an , reflecting the escalating complexity of regulatory frameworks. Furthermore, organizations that prioritize life sciences compliance are better positioned to navigate challenges, as evidenced by the 95% rise in regulatory spending noted in 2023. Establishing a robust regulatory framework not only shields against penalties but also fosters trust and accountability within the organization.
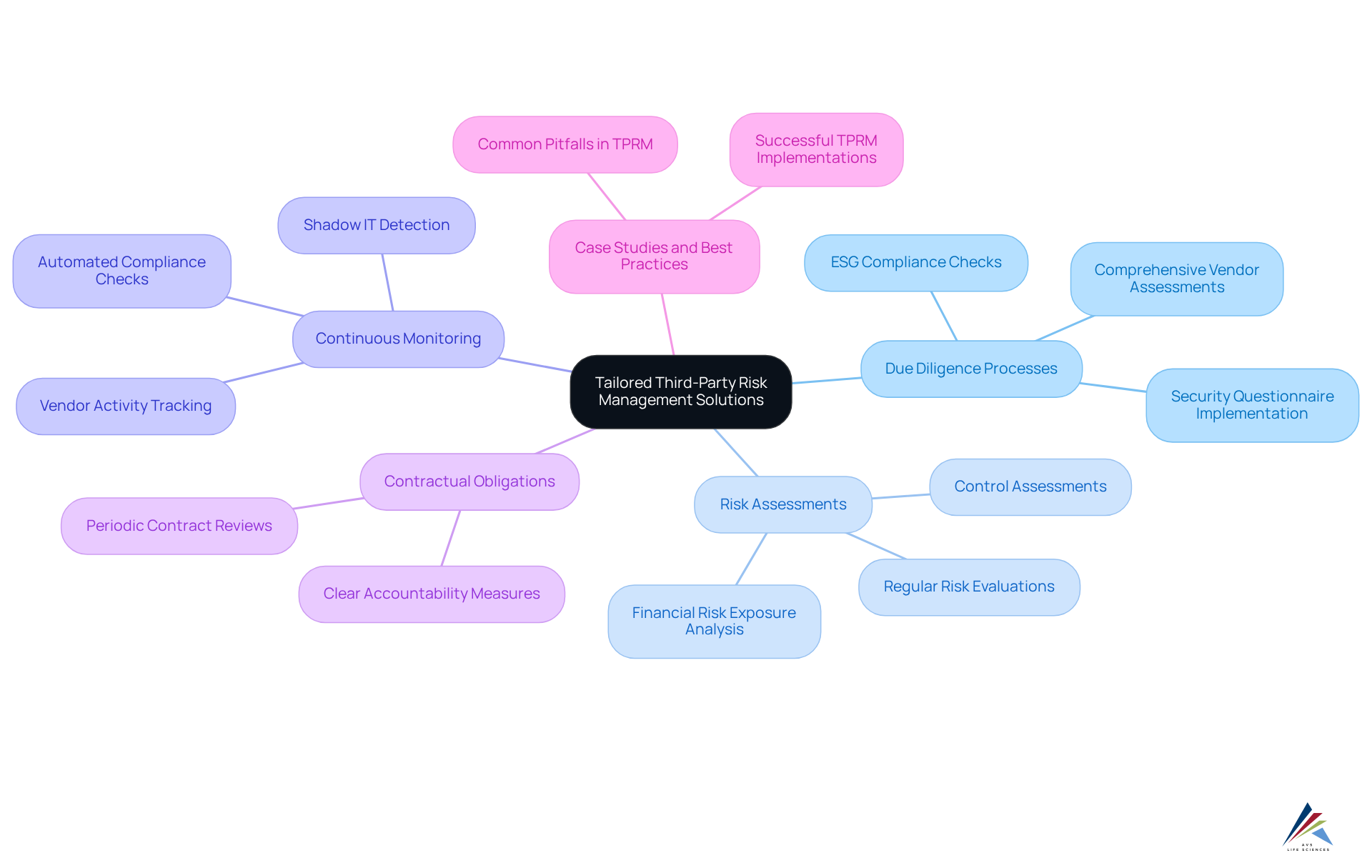
Implement Tailored Third-Party Risk Management Solutions
In the life sciences compliance sector, organizations often rely on third-party vendors for critical services, including manufacturing and clinical trials. However, these collaborations can pose significant challenges, such as regulatory violations and data breaches. To effectively mitigate these risks, companies must establish a tailored third-party risk management (TPRM) program that includes:
Clear contractual obligations regarding adherence and data security are essential to ensure accountability. According to the EY 2023 Global Third-Party Risk Management Survey, 90% of entities view TPRM as an increasing priority, underscoring its growing importance in safeguarding operations and meeting regulatory standards. By actively managing third-party challenges, organizations can enhance their resilience and ensure life sciences compliance with evolving regulatory demands.
Incorporating successful case studies that showcase effective third-party management practices can offer valuable insights into the implementation of TPRM programs. Moreover, addressing common pitfalls in TPRM implementation can help organizations avoid missteps and establish robust risk management strategies.
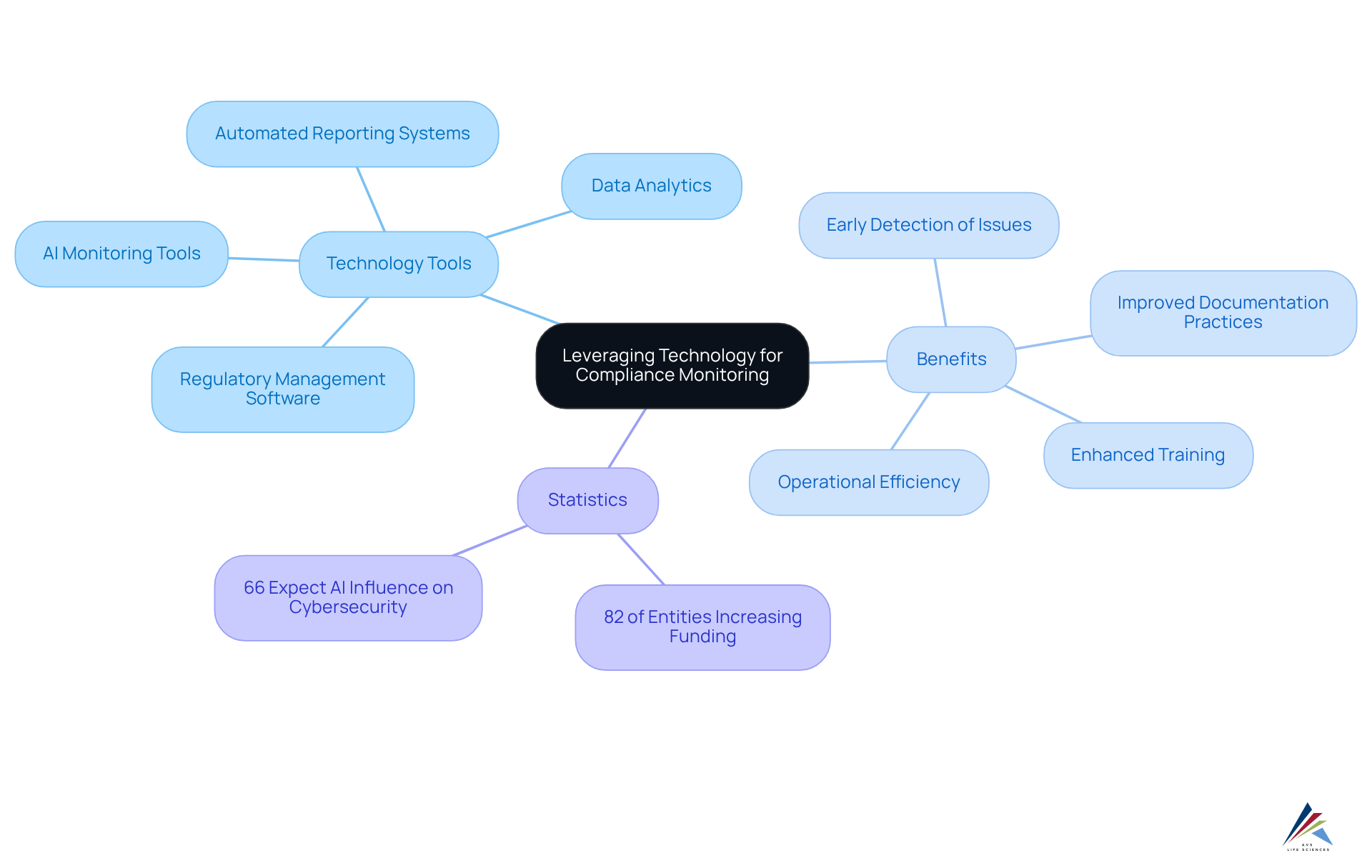
Leverage Technology to Enhance Compliance Monitoring
Incorporating technology into regulatory oversight procedures is essential for entities striving to uphold strong adherence in the life sciences field. Regulatory management software, automated reporting systems, and data analytics platforms provide real-time insights into regulatory status, enabling early detection of potential issues. For instance, AI-powered monitoring tools can identify irregularities in data that may indicate regulatory risks, allowing businesses to address them proactively. AVS Life Sciences underscores the importance of adhering to GXP standards and FDA regulations, ensuring that entities maintain high-quality documentation practices and effective Standard Operating Procedures (SOPs).
Based on a recent survey, 82% of entities intend to increase funding for technology in regulatory activities, highlighting the growing recognition of its significance. Furthermore, digital platforms enhance training and communication among staff, ensuring that all employees are informed of regulatory requirements and updates. As Lucia Giles, Sr. Content Marketing Manager, noted, "66% of entities anticipate AI to exert the greatest influence on cybersecurity in the upcoming year, yet only 37% have procedures established to evaluate the security of AI tools prior to deployment."
By leveraging these technologies, including AVS Life Sciences' expert solutions in GMP compliance and validation, entities can establish a resilient compliance framework that adapts to the ever-evolving regulatory landscape. This approach ultimately improves and reduces the risk of non-compliance. Additionally, organizations that implemented AI and automation reported significantly lower data breach costs, underscoring the effectiveness of these tools in real-world scenarios.
Conclusion
Navigating the intricate landscape of life sciences compliance is essential for organizations striving to meet regulatory standards and maintain quality assurance. Understanding the regulatory framework, defining the roles of compliance officers, implementing tailored third-party risk management solutions, and leveraging technology are critical strategies for success in this field. Each of these components fosters a culture of compliance that mitigates risks and enhances operational integrity.
Key insights include:
- The necessity of continuous training for staff to stay updated on regulatory changes.
- The pivotal role of compliance officers in developing robust programs.
- The increasing importance of third-party risk management in safeguarding operations.
- Integrating technology into compliance monitoring as a powerful tool for early detection of potential issues, ultimately improving efficiency and reducing the risk of non-compliance.
Organizations in the life sciences sector must prioritize compliance as a cornerstone of their operational strategy. By adopting these best practices, entities can effectively navigate the complexities of regulatory requirements while building a resilient framework that fosters trust and accountability. Embracing these strategies equips organizations to face the evolving challenges of the industry, contributing to better outcomes for all stakeholders involved.
Frequently Asked Questions
What is the regulatory landscape in life sciences?
The regulatory landscape in life sciences involves navigating a complex framework of regulations that differ by region and product type, focusing on compliance with standards such as Good Manufacturing Practices (GMP), ISO specifications, and Quality System Regulations (QSR).
What are Good Manufacturing Practices (GMP)?
Good Manufacturing Practices (GMP) are regulations that ensure products are consistently produced and controlled according to stringent quality criteria.
What role do ISO specifications play in life sciences compliance?
ISO specifications provide comprehensive guidelines for developing efficient quality management systems within the life sciences industry.
What are Quality System Regulations (QSR)?
Quality System Regulations (QSR) outline specific requirements for the manufacturing and quality control of medical devices.
Why is it important to stay updated on life sciences compliance?
Staying updated on life sciences compliance is crucial because non-compliance can result in severe consequences, including substantial fines and product recalls.
How can organizations ensure adherence to life sciences regulations?
Organizations can ensure adherence by conducting regular training and updates for staff to align operational processes with current regulatory criteria.
