Master Integration with ERP, MES, and QMS for Compliance Success

Overview
This article delves into the pathway to compliance success through the strategic integration of ERP (Enterprise Resource Planning), MES (Manufacturing Execution Systems), and QMS (Quality Management Systems). It asserts that a profound understanding of the core functions of these systems, coupled with adherence to a structured integration process, can significantly bolster operational efficiency and ensure compliance with regulatory standards, particularly within the pharmaceutical sector.
By addressing the compliance challenges faced by organizations, this article outlines detailed solutions that can lead to enhanced operational capabilities. Ultimately, it provides actionable insights that encourage engagement with AVS Life Sciences, emphasizing the critical role of these systems in navigating the complexities of compliance.
Introduction
In a rapidly evolving landscape where regulatory compliance is non-negotiable, the integration of ERP, MES, and QMS systems emerges as a pivotal strategy for organizations, particularly in the pharmaceutical sector. This article delves into the essential frameworks governing business processes, manufacturing execution, and quality management, revealing how their synergy can enhance operational efficiency and ensure adherence to stringent regulations.
However, the journey towards seamless integration is fraught with challenges. What are the key steps to navigate these complexities and unlock the full potential of these systems?
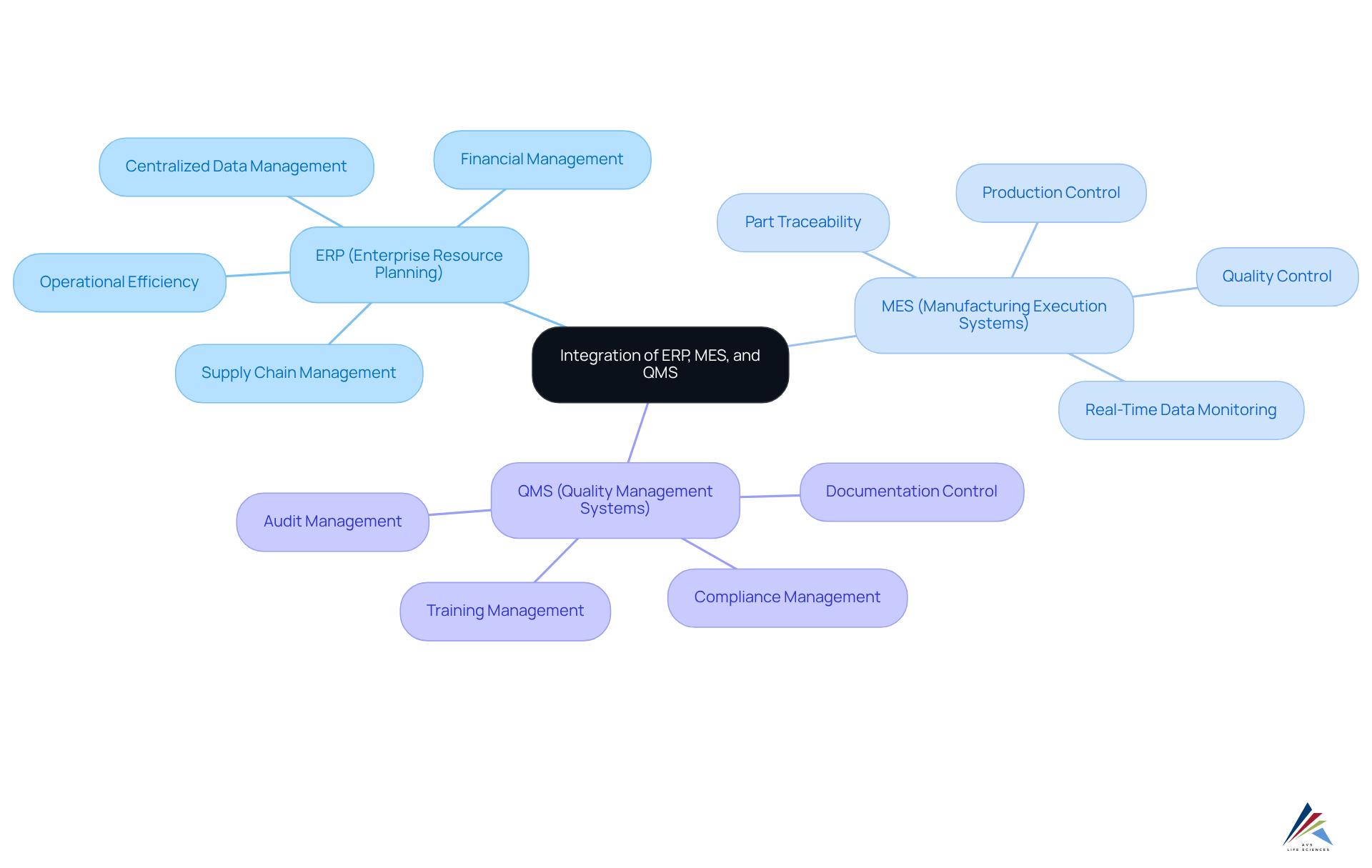
Understand ERP, MES, and QMS Fundamentals
To effectively integrate ERP, MES, and QMS, it is crucial to grasp their core functions:
- ERP (Enterprise Resource Planning): This system oversees business processes across various departments, including finance, HR, and supply chain. By offering a centralized platform for data management, ERP enhances decision-making and operational efficiency—essential elements in the pharmaceutical industry where adherence to regulations and accuracy are paramount.
- MES (Manufacturing Execution Systems): MES focuses on the manufacturing process, delivering real-time data on production activities. It plays a pivotal role in monitoring and controlling production, ensuring that operations align with business objectives and compliance standards. For instance, manufacturers utilizing MES can achieve significant enhancements in production efficiency and quality control, as evidenced by organizations that have integrated MES with ERP solutions.
- QMS (Quality Management Systems): QMS is vital for managing quality processes and ensuring compliance with regulatory requirements. It encompasses documentation, training, and audits, which are critical for maintaining product quality and safety in the pharmaceutical sector. Effective QMS implementation can lead to zero findings during audits, underscoring the importance of quality in compliance-driven environments.
Understanding the functions and interconnections of these frameworks is the initial step toward with ERP, MES, and QMS. Companies that have effectively achieved integration with ERP, MES, and QMS report enhanced operational efficiency and improved quality management, illustrating the transformative potential of these systems when aligned correctly. This combination is particularly crucial in the pharmaceutical industry, where regulatory compliance and product integrity are non-negotiable.
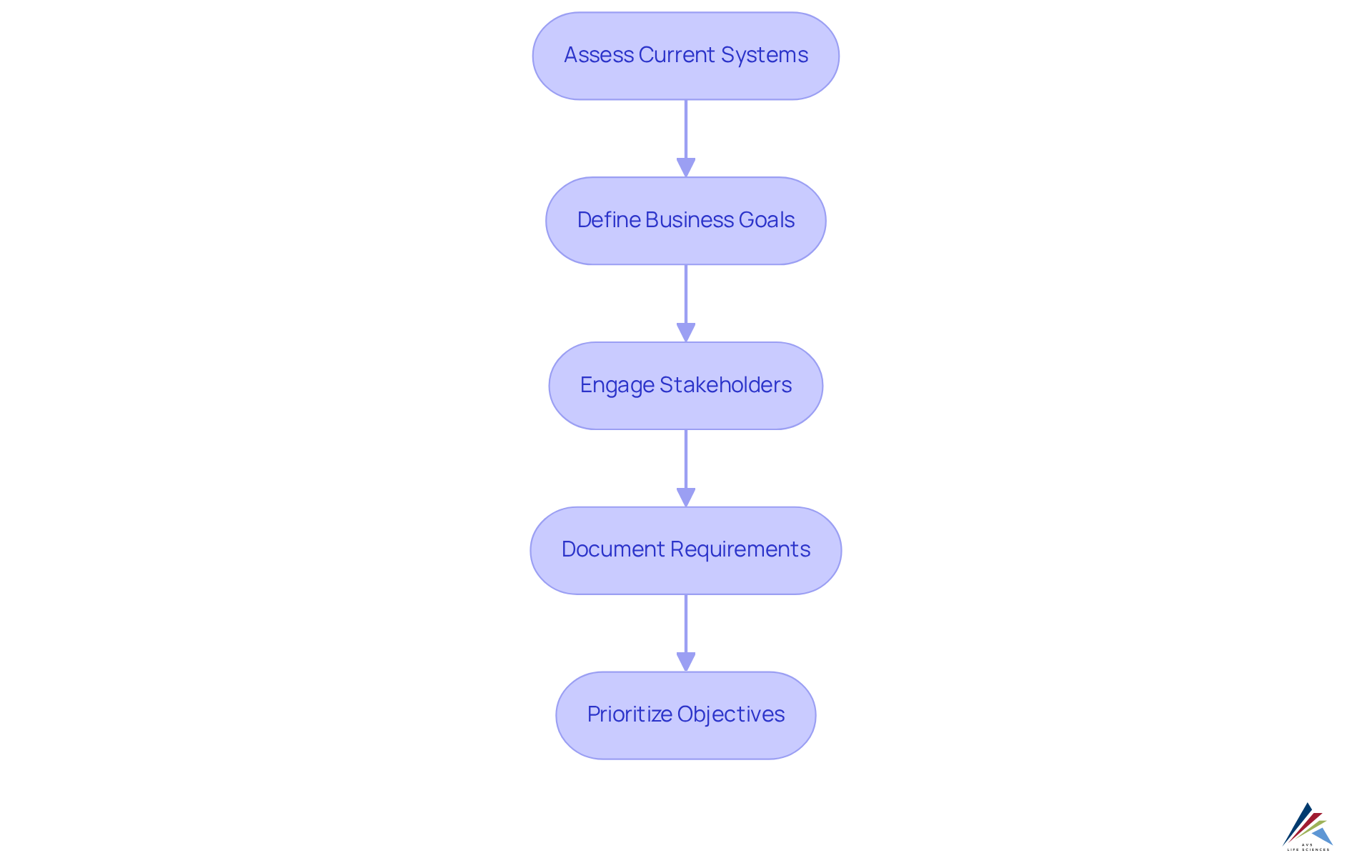
Identify Integration Requirements and Objectives
To effectively identify integration requirements and objectives, consider the following steps:
- Assess Current Systems: Conduct a thorough evaluation of your existing systems with a focus on their integration with ERP, MES, and QMS to understand their strengths and weaknesses. This evaluation will assist in that require attention during the merging process. Notably, 50% of ERP implementations fail in their initial attempt, highlighting the importance of this step.
- Define Business Goals: Clearly articulate the business objectives you aim to achieve through integration. These objectives may involve improving data accuracy, enhancing adherence to regulations, or streamlining operations to boost overall efficiency. As Rahat Ahmed, Resource Management Growth Leader, states, "Plan with precision, meet compliance requirements, and build high-performing teams."
- Engage Stakeholders: Actively involve key stakeholders from various departments, such as IT, production, and quality assurance. Collecting insights from these groups will ensure that the incorporation meets their needs and expectations, fostering a collaborative environment. A case study on "Challenges in ERP Implementation" illustrates that inadequate stakeholder engagement can lead to project delays and failures.
- Document Requirements: Create a thorough list of functional and technical specifications for the amalgamation. This documentation should encompass data exchange protocols, user access levels, and compliance standards that must be adhered to throughout the process.
- Prioritize Objectives: Rank the identified objectives based on their significance and feasibility. This prioritization will direct the unification process, ensuring that resources are allocated effectively to achieve the most critical goals. Current trends suggest a shift towards integration with ERP, MES, and QMS in cloud-based solutions for combination needs in 2025, which should be taken into account during this phase.
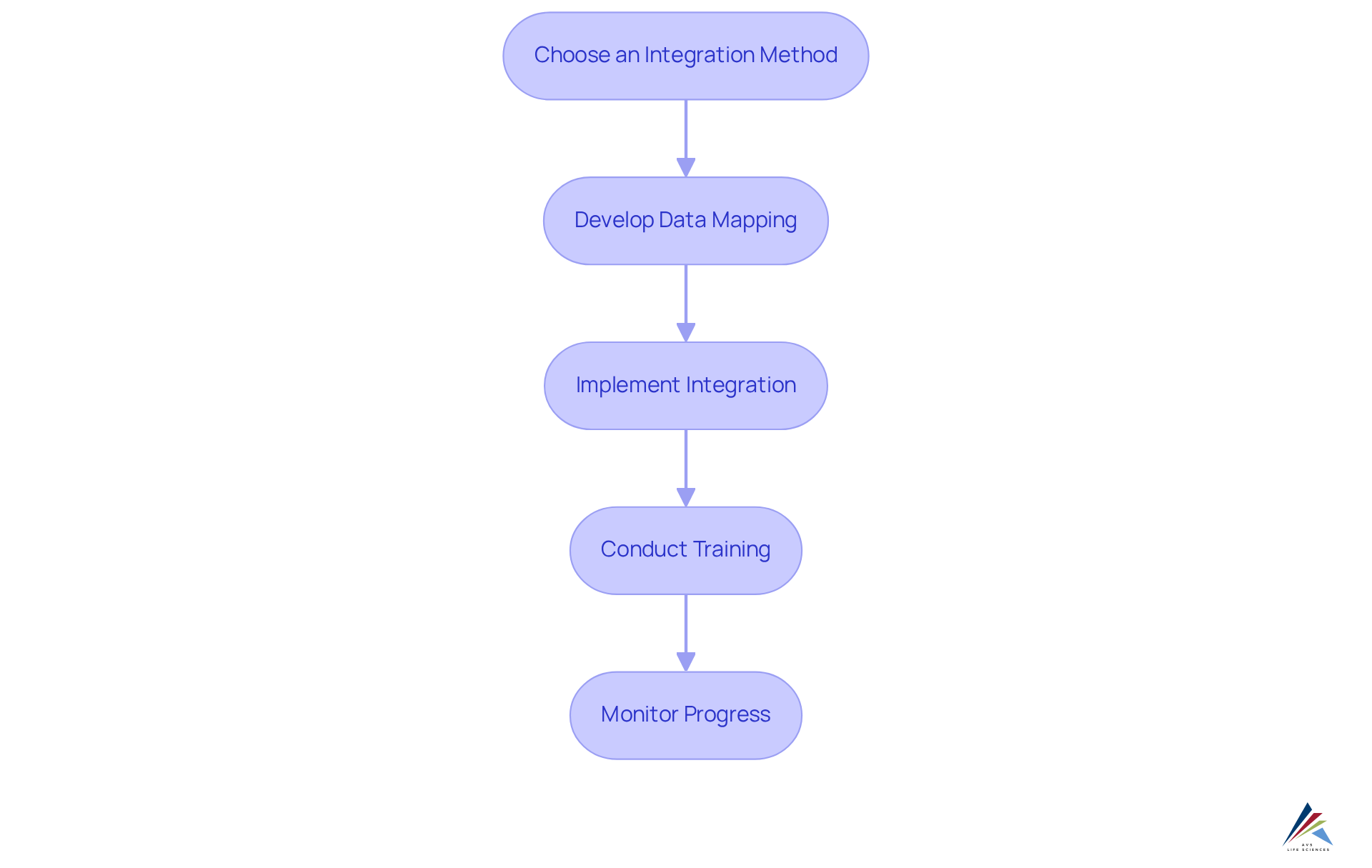
Execute the Integration Process
To effectively execute the integration process for ERP, MES, and QMS systems, particularly in the context of AVS Life Sciences' comprehensive quality management and regulatory compliance solutions, follow these essential steps:
-
Choose an Integration Method: Select the integration approach that aligns with your organization's needs. Common methods include:
- API Integration: Utilizing application programming interfaces to facilitate seamless connections between systems—a method endorsed by AVS Life Sciences for its efficiency.
- Middleware Solutions: Implementing software that acts as a bridge, enabling communication between distinct platforms, which AVS Life Sciences has successfully utilized in various projects.
- Custom Development: Crafting tailored solutions designed to address specific integration requirements, reflecting AVS Life Sciences' commitment to personalized service.
-
Develop Data Mapping: Establish a clear data flow between the platforms. Create a comprehensive data mapping document that specifies how data fields in the ERP correspond to those in the MES and QMS, ensuring consistency and accuracy—a practice emphasized by AVS Life Sciences in their methodologies.
-
Implement Integration: Initiate the technical implementation phase. This entails setting up software, creating data transfer protocols, and ensuring that all networks can communicate effectively and efficiently, as illustrated in AVS Life Sciences' successful case studies.
-
Conduct Training: Provide thorough training for users on navigating the integrated platforms. Ensure they are equipped to access and utilize the data effectively, enhancing operational efficiency—a key focus area for AVS Life Sciences.
-
Monitor Progress: Continuously oversee the merging process to identify any potential issues or bottlenecks. Make essential modifications to enable a seamless transition and enhance performance—a strategy that AVS Life Sciences uses to guarantee client satisfaction.
Statistics suggest that organizations can significantly improve operational efficiency and compliance through the integration with ERP, MES, and QMS using middleware solutions. For instance, the MES market is projected to grow at a CAGR of 11.07% from 2022 to 2027, highlighting the increasing importance of these systems in the industry. API incorporation, in particular, has proven to be a critical enabler in the life sciences sector, facilitating real-time data exchange and enhancing decision-making processes. Successful methods of integration with ERP / MES / QMS employed by pharmaceutical companies, including those highlighted by AVS Life Sciences, often demonstrate the effectiveness of these strategies in achieving compliance and operational excellence.
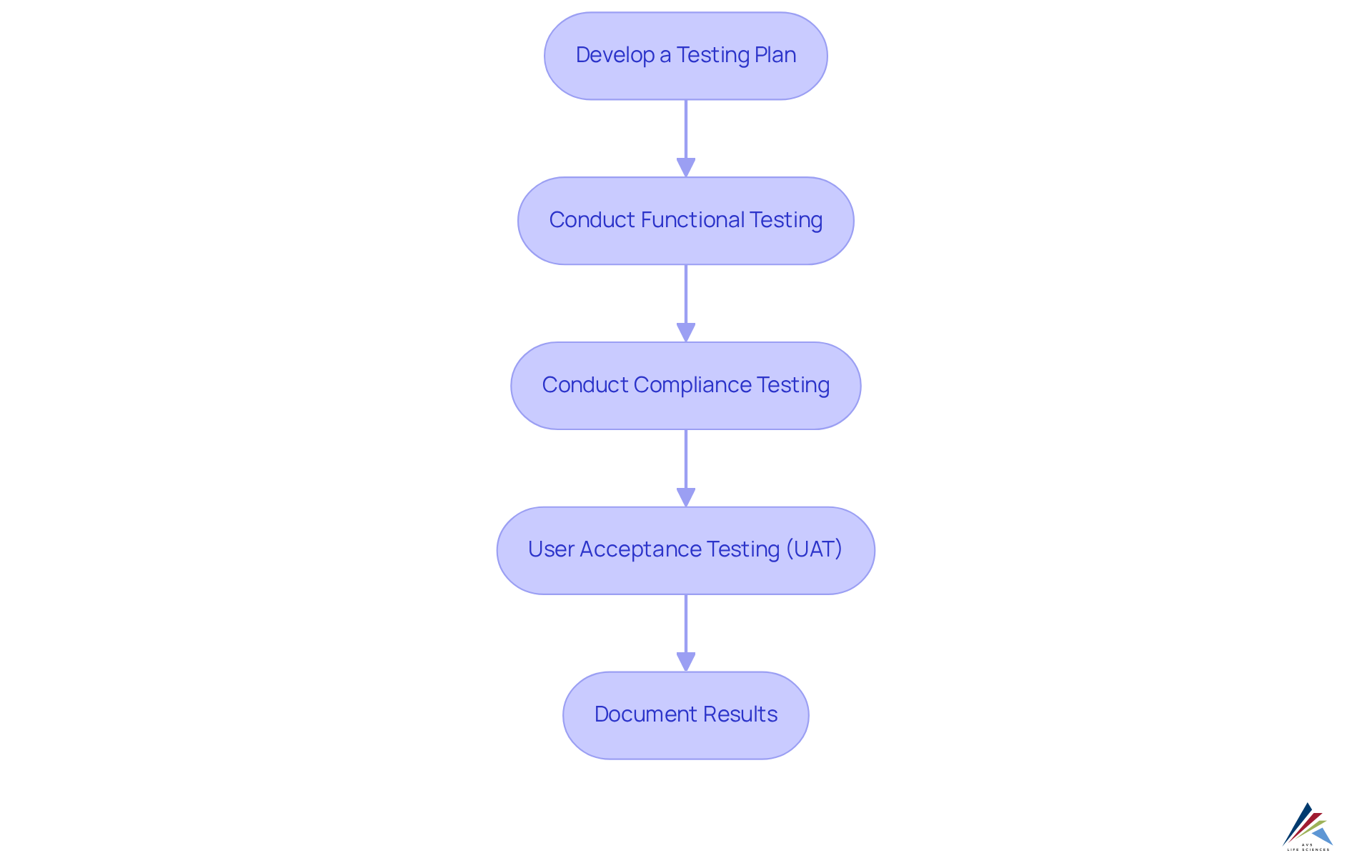
Validate and Test the Integrated Systems
To effectively validate and test the integrated systems, it is crucial to adhere to the following steps:
- Develop a Testing Plan: Formulate a detailed testing strategy that specifies the scope, objectives, and methodologies for assessing the integrated frameworks. This plan should align with compliance benchmarks and operational needs.
- Conduct Functional Testing: Execute tests on each function of the integrated frameworks to confirm they operate as intended. This encompasses verifying data transfers, user access, and responses, ensuring seamless integration with ERP / MES / QMS.
- Conduct Compliance Testing: Confirm that the integrated setups meet all pertinent regulatory requirements, such as Good Manufacturing Practices (GMP) and ISO standards. This process may involve thorough documentation reviews and compliance audits to ensure adherence to industry regulations.
- : Involve end-users in the testing procedure to gather insights on the usability and functionality of the application. UAT acts as an essential stage to pinpoint any gaps or problems, facilitating modifications that improve user satisfaction and operational efficiency. Engaging end-users promotes a feeling of ownership and enhances the chances of successful acceptance of the new solution.
- Document Results: Maintain comprehensive records of all testing outcomes, including any issues encountered and their resolutions. This documentation is essential for compliance verification and acts as a reference for future audits and improvements.
Maintain and Optimize the Integrated Solution
To maintain and optimize the integrated solution, it is essential to follow these steps:
- Regularly Review Performance Metrics: Continuously monitor the performance of integrated networks using key performance indicators (KPIs) to identify areas for improvement. This proactive approach ensures that systems operate at .
- Conduct Periodic Audits: Schedule regular audits to confirm adherence to regulatory standards and internal policies. Statistics indicate that organizations performing regular regulatory audits save an average of $2.86 million. Address any findings promptly to uphold compliance and mitigate risks.
- Gather User Feedback: Actively collect feedback from users to pinpoint pain points and areas for enhancement. This feedback loop is essential for guiding future updates and enhancements, ensuring that the framework evolves to effectively meet user needs.
- Stay Informed on Regulations: Remain vigilant about changes in regulatory requirements that may impact your operations. Modify procedures and documentation as needed to guarantee ongoing adherence, as the landscape of regulations is continually evolving.
- Invest in Training: Offer continuous training for users to keep them updated on enhancements and best practices. This investment not only improves operational efficiency but also fosters a culture of adherence, ensuring that all users are prepared to manage the intricacies of regulatory requirements effectively.
Regular audits are crucial for ensuring adherence, as they assist organizations in recognizing and resolving problems before they escalate. The significance of these audits cannot be overstated; they serve as a critical component in safeguarding against non-compliance risks. In the pharmaceutical industry, performance optimization examples include the integration with ERP / MES / QMS systems, which streamline operations and enhance data accuracy, ultimately leading to improved compliance outcomes.
Conclusion
Mastering the integration of ERP, MES, and QMS systems is essential for achieving compliance success in the pharmaceutical industry. Understanding the distinct roles of these systems and their interconnections enables organizations to streamline operations, enhance data accuracy, and ensure adherence to stringent regulatory standards. This integration not only facilitates better decision-making but also significantly improves operational efficiency and quality management.
This article outlines a comprehensive approach to effective integration, beginning with:
- An assessment of current systems and the definition of business goals
- The execution of the integration process
- Validation of results
Engaging stakeholders throughout the process and prioritizing objectives are crucial steps that lay the groundwork for a successful integration. Furthermore, continuous monitoring and optimization of the integrated systems ensure that organizations remain compliant and responsive to the ever-evolving regulatory landscape.
Ultimately, the integration of ERP, MES, and QMS transcends mere technical endeavor; it represents a strategic imperative that can lead to substantial improvements in compliance and operational excellence. Organizations are urged to embrace these systems and invest in their integration, as the benefits extend far beyond compliance. They foster a culture of quality and efficiency that is vital for long-term success in the highly regulated pharmaceutical sector.
Frequently Asked Questions
What are the core functions of ERP, MES, and QMS?
ERP (Enterprise Resource Planning) oversees business processes across various departments, enhancing decision-making and operational efficiency. MES (Manufacturing Execution Systems) focuses on real-time data for production activities, ensuring alignment with business objectives and compliance standards. QMS (Quality Management Systems) manages quality processes and compliance with regulatory requirements, encompassing documentation, training, and audits.
Why is understanding ERP, MES, and QMS important for integration?
Understanding the functions and interconnections of ERP, MES, and QMS is crucial for successful integration. Companies that achieve effective integration report enhanced operational efficiency and improved quality management, which is especially important in the pharmaceutical industry where regulatory compliance and product integrity are critical.
What steps should be taken to identify integration requirements and objectives?
To identify integration requirements and objectives, one should assess current systems, define business goals, engage stakeholders, document requirements, and prioritize objectives.
How can assessing current systems help in integration?
Conducting a thorough evaluation of existing systems helps identify strengths and weaknesses, revealing gaps that require attention during the merging process. This step is vital as 50% of ERP implementations fail in their initial attempts.
What role do business goals play in the integration process?
Clearly articulating business objectives helps guide the integration process, focusing on improving data accuracy, enhancing regulatory adherence, and streamlining operations for increased efficiency.
Why is stakeholder engagement important in the integration process?
Engaging key stakeholders from various departments ensures that the integration meets their needs and expectations, fostering collaboration and reducing the risk of project delays and failures.
What should be included in the documentation of integration requirements?
Documentation should include a thorough list of functional and technical specifications, such as data exchange protocols, user access levels, and compliance standards that need to be adhered to throughout the integration process.
How should objectives be prioritized during integration?
Objectives should be ranked based on their significance and feasibility, guiding the unification process and ensuring effective allocation of resources to achieve the most critical goals.
