Master Installation Qualification: Key Steps for Compliance Success

Overview
Mastering Installation Qualification (IQ) in the pharmaceutical sector is crucial for compliance and operational integrity. The essential steps include:
- Thorough documentation preparation
- Pre-installation checks
- Meticulous equipment installation
- Functional testing
- Compiling a validation assessment report
These steps not only ensure adherence to regulatory standards but also highlight the importance of rigorous protocols. Inadequate documentation can lead to significant consequences during GMP audits, underscoring the necessity for meticulous attention to detail in every phase of the process.
Introduction
Ensuring compliance in the pharmaceutical industry hinges on robust validation processes, with Installation Qualification (IQ) serving as a critical foundation. This guide delves into the essential steps and best practices for mastering IQ, illuminating how organizations can enhance operational integrity while adhering to stringent regulatory standards.
With the increasing complexities in compliance requirements, what are the most effective strategies to navigate these challenges and ensure a successful installation qualification? This exploration promises to equip compliance officers with actionable insights and proven methods to excel in their roles.
Understand Installation Qualification in Pharma
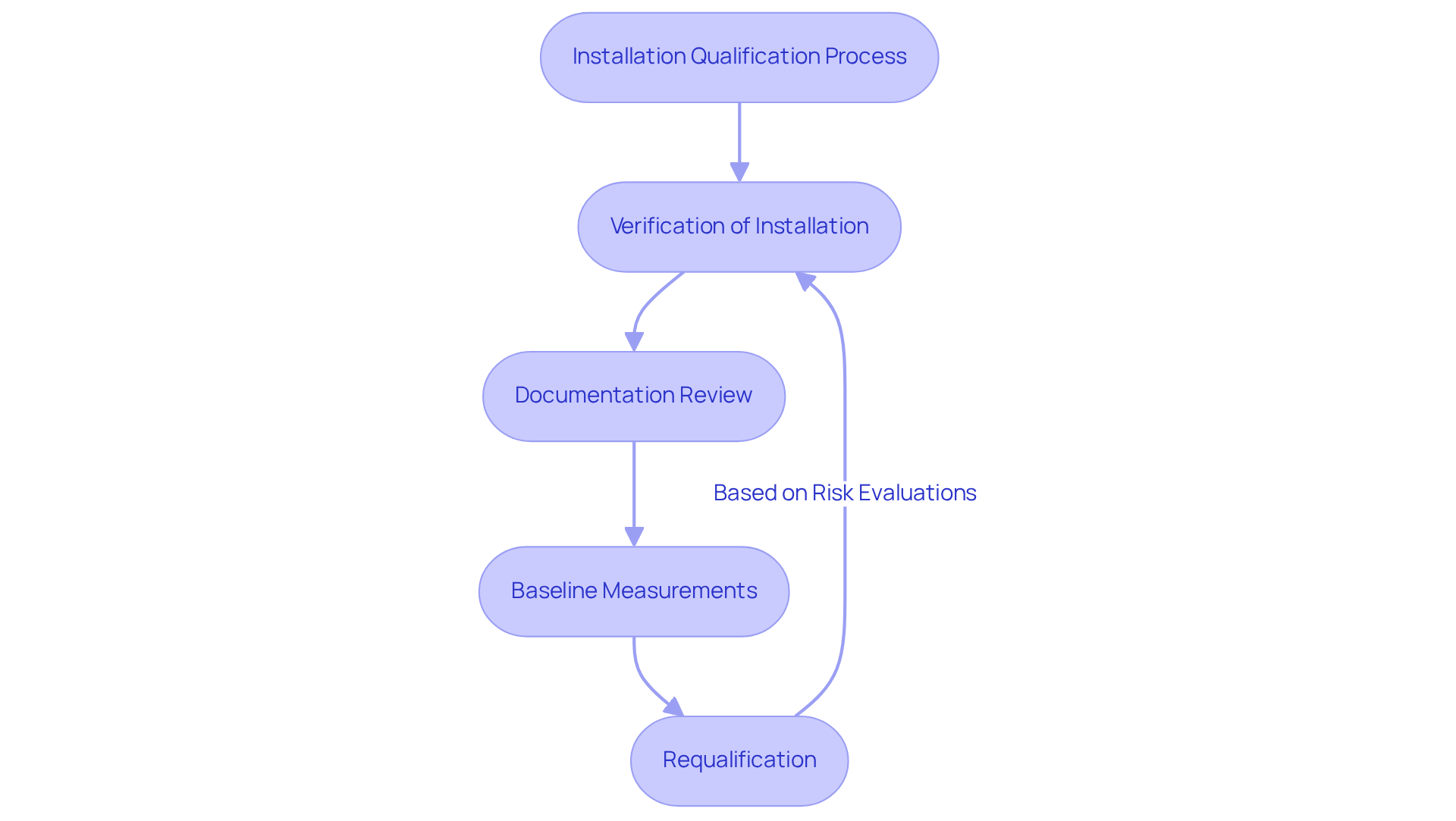
Qualification of equipment serves as a vital element of the validation framework within the pharmaceutical sector, acting as the foundation for ensuring adherence to , including GXP and FDA guidelines. This process verifies that equipment and systems are installed correctly and function as intended. The primary objectives of include:
- Verification of Installation: This critical step ensures that all components are installed according to the manufacturer's specifications and design requirements, which is essential for maintaining operational integrity.
- Documentation Review: A thorough review of all necessary documentation, including installation manuals and specifications, is conducted to confirm accuracy and completeness. This documentation is not only essential for regulatory adherence but also serves as a vital reference for future operations, aligning with standard operating procedures (SOPs).
- Baseline Measurements: Establishing is crucial for comparing against future validation phases. These metrics facilitate the assessment of ongoing reliability and effectiveness of the equipment.
Moreover, it is imperative to recognize that requalification should be conducted regularly based on a risk evaluation and to ensure continuous adherence. For less complex systems, IQ, OQ, and PQ activities can be consolidated into a single document, offering flexibility in documentation practices.
The significance of installation qualification extends beyond mere compliance; it is integral to the overall . For instance, AVS Life Sciences recently initiated a validation project for an , demonstrating how effective IQ practices can streamline operations and enhance production efficiency by eliminating unnecessary steps. The APCS featured three Programmable Logic Controllers (PLCs) and five Human-Machine Interfaces (HMIs), illustrating the project's complexity and its impact on . Such real-world applications underscore the necessity of rigorous installation qualification protocols in order to achieve consistent results and uphold .
As industry leaders emphasize, the validation steps—Installation Qualification (IQ), (IV), Operational Verification (OV), and Performance Verification (PV)—are essential for confirming that equipment and systems are installed and functioning correctly. Looking ahead to 2025, the importance of Setup Verification in pharmaceutical compliance remains paramount, as organizations strive to navigate increasingly intricate regulatory environments while ensuring product quality and safety.
Follow Key Steps for Installation Qualification
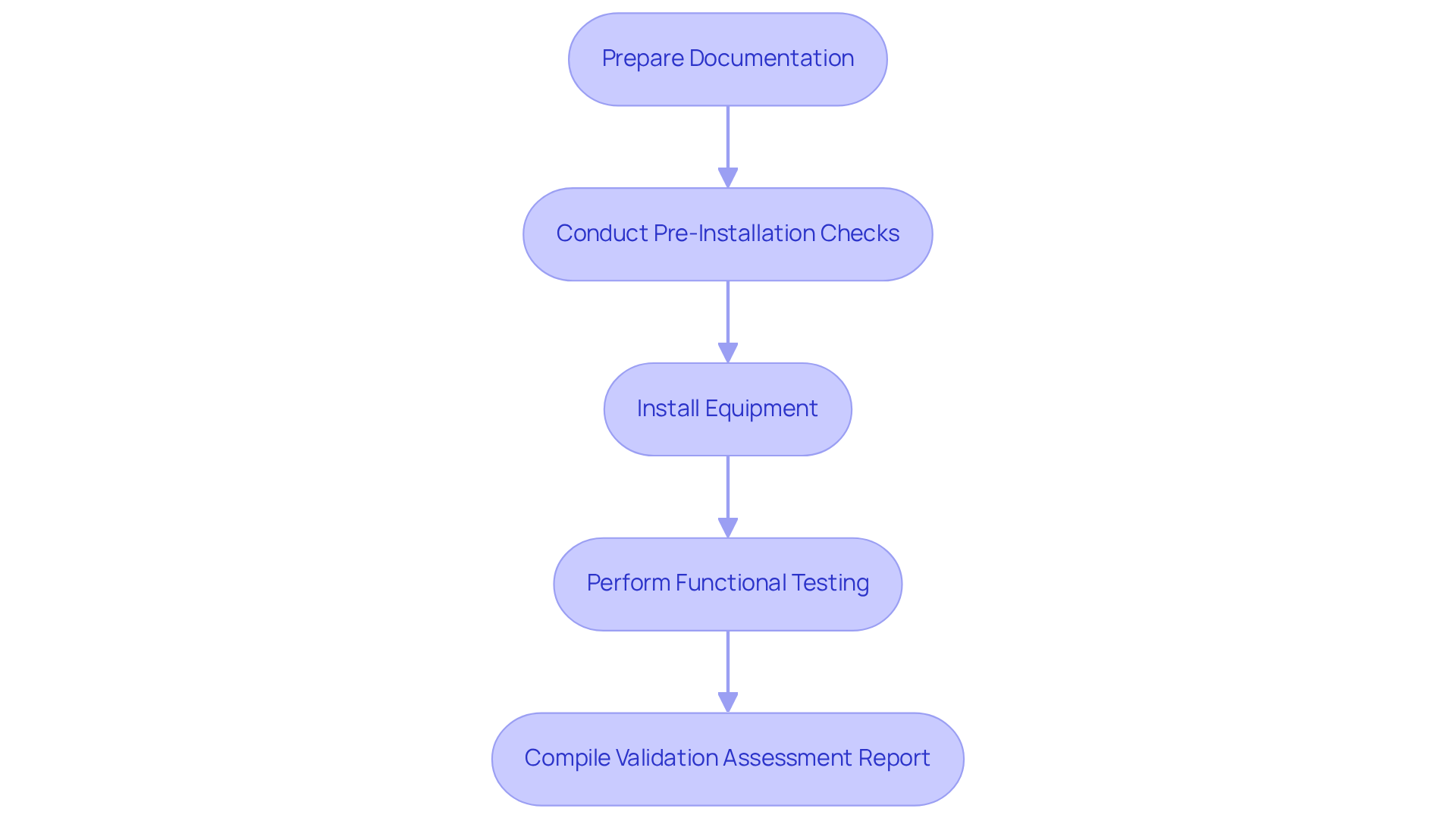
To successfully complete an , it is essential to follow these :
- Prepare Documentation: Gather all relevant installation manuals, specifications, and drawings. Ensure that you have the latest versions and that they are accessible.
- Conduct Pre-Installation Checks: Verify that the installation site meets environmental and operational requirements, including power supply, temperature, and humidity controls.
- Install Equipment: Follow the manufacturer's installation instructions meticulously. Ensure that all components are installed correctly and securely.
- Perform : After installation, conduct functional tests to confirm that the equipment operates as intended. Document any discrepancies and corrective actions taken.
- Compile : Document all findings, including successful tests and any problems faced. This report serves as an official record of the installation qualification procedure and is crucial for .
By adhering to these steps, you can guarantee a .
Ensure GMP Compliance Through Installation Qualification
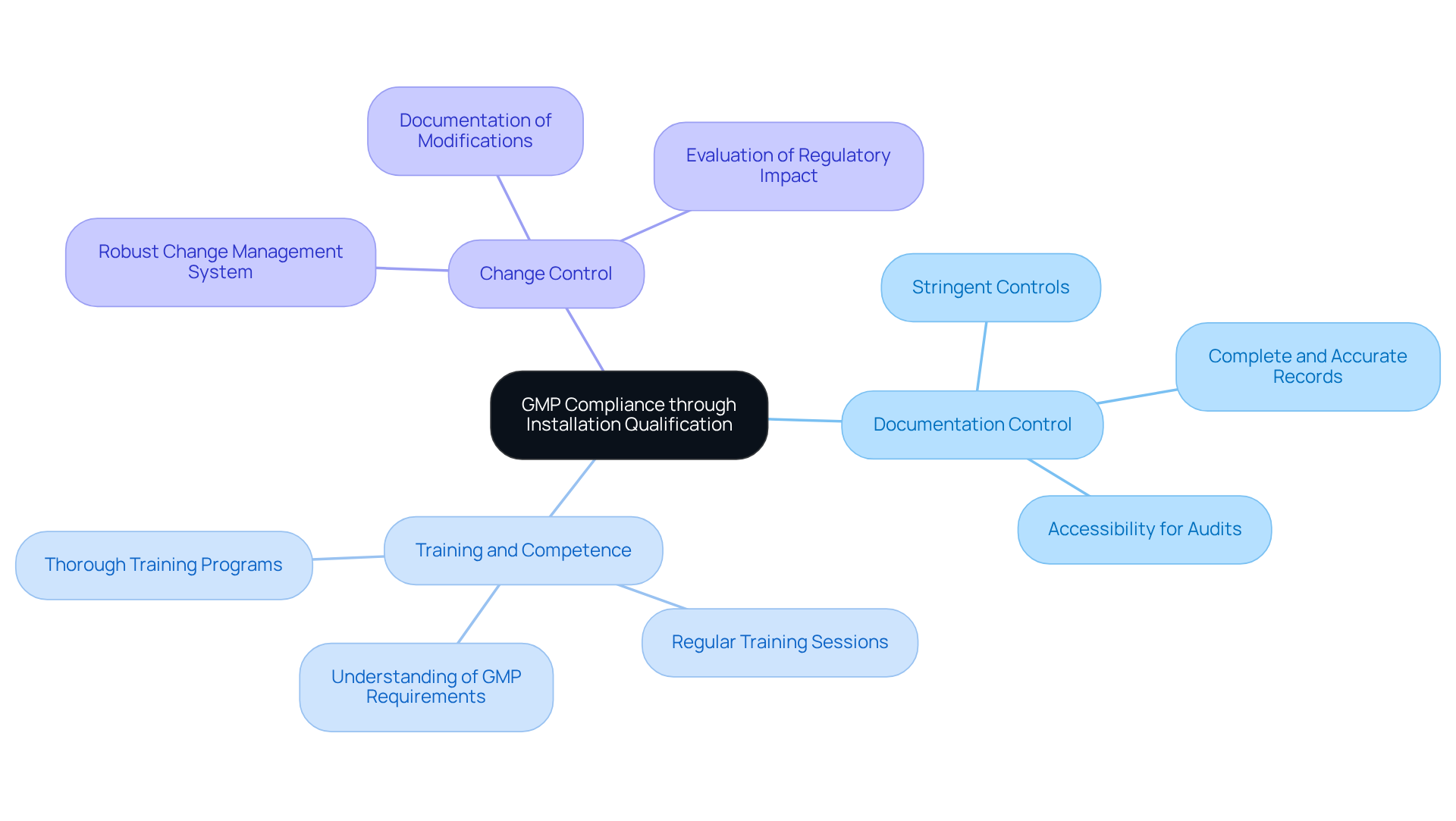
To ensure that your Installation Qualification (IQ) aligns with , it is essential to focus on several key areas:
- : Implement stringent controls over all documentation related to the IQ process. Records must be complete, accurate, and easily accessible for audits. Inadequate documentation is a common reason for GMP audit failures, with studies indicating that over 45% of companies face issues due to poor documentation practices. offers comprehensive to help organizations maintain proper documentation.
- : Personnel involved in the IQ procedure must be thoroughly trained and skilled in their roles. This encompasses a thorough understanding of the equipment, the IQ procedure, and GMP requirements. Regular training sessions can significantly lower the risk of , supported by AVS Life Sciences' expert consulting services.
- Change Control: Establish a robust to oversee any modifications to equipment or installation procedures. This guarantees that all alterations are recorded and evaluated for their effect on regulations, thereby preserving the integrity of the installation qualification process. AVS Life Sciences can assist in developing effective change control strategies.
Regular reviews and audits should be conducted frequently on the to identify areas for enhancement and ensure ongoing adherence to GMP standards. These proactive steps not only improve adherence but also promote a culture of ongoing enhancement within the organization, with direction from AVS Life Sciences' demonstrated excellence in .
Incorporating these methods into your setup validation process is essential for improving adherence and reducing risks linked to regulatory reviews. By prioritizing documentation control and ensuring that personnel are well-trained, organizations can significantly improve their chances of passing GMP audits and maintaining high-quality standards.
Create an Effective Installation Qualification Protocol
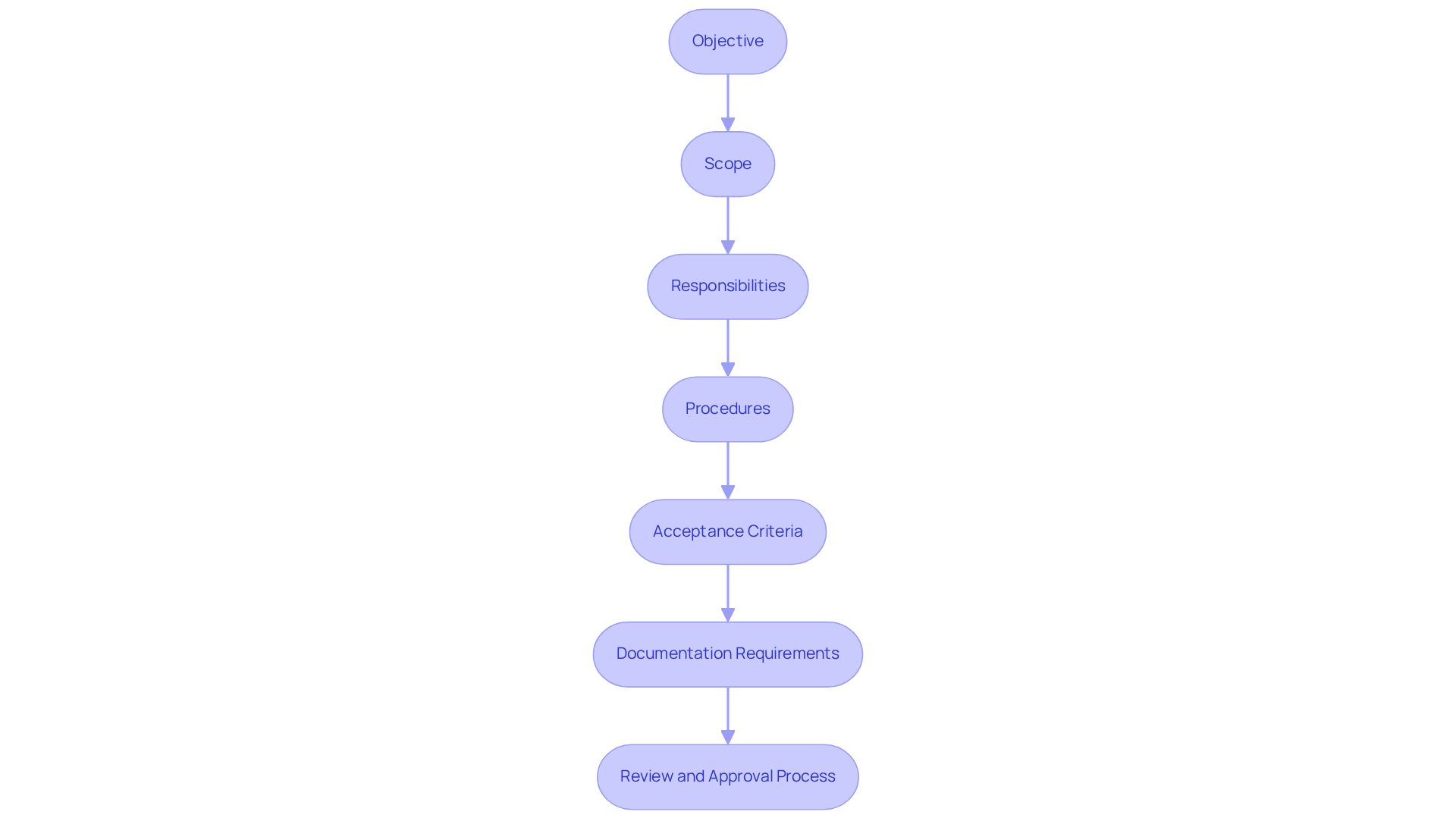
To create an effective , it is essential to incorporate the following elements:
- Objective: Clearly articulate the purpose of the IQ, outlining what it aims to achieve in terms of adherence and operational readiness, in alignment with the .
- Scope: Define the scope of the IQ, specifying the equipment and systems that will be covered, ensuring .
- Responsibilities: Outline the roles and duties of all personnel engaged in the IQ procedure, ensuring accountability and clarity in execution.
- Procedures: Provide for conducting the IQ, including pre-installation checks, installation steps, and functional testing to verify compliance with specifications.
- : Specify the criteria that must be met for the IQ to be deemed successful, ensuring that all necessary standards are satisfied.
- Documentation Requirements: List all throughout the IQ procedure, which is crucial for traceability and audit readiness.
- : Describe the process for reviewing and approving the IQ protocol and the final IQ report, ensuring that all steps are validated and compliant with industry standards.
Incorporating these elements into your installation qualification protocol not only ensures a but also aligns with the V-Model, enhancing compliance and operational efficiency.
Conclusion
Mastering Installation Qualification (IQ) is essential for ensuring compliance within the pharmaceutical industry. This process not only verifies the correct installation of equipment but also reinforces adherence to regulatory standards, thereby safeguarding product quality and safety. A comprehensive understanding of IQ is crucial for organizations aiming to navigate the complexities of regulatory requirements while maintaining operational efficiency.
Key steps in the Installation Qualification process include:
- Thorough documentation
- Pre-installation checks
- Meticulous installation
- Functional testing
- Compiling a validation assessment report
Each of these steps plays a vital role in establishing a robust framework that aligns with Good Manufacturing Practices (GMP). Additionally, focusing on documentation control, personnel training, and change management enhances compliance and reduces the risk of audit failures.
The importance of a well-structured IQ protocol cannot be overstated. By incorporating clear objectives, defined scopes, detailed procedures, and stringent acceptance criteria, organizations can ensure a successful installation qualification process. As the pharmaceutical landscape continues to evolve, prioritizing these best practices will not only facilitate compliance but also foster a culture of continuous improvement. Taking proactive measures today will position organizations for success in meeting the regulatory challenges of tomorrow.
Frequently Asked Questions
What is Installation Qualification (IQ) in the pharmaceutical sector?
Installation Qualification (IQ) is a process that verifies that equipment and systems are installed correctly and function as intended, serving as a foundation for ensuring adherence to regulatory standards, including GXP and FDA guidelines.
What are the primary objectives of Installation Qualification?
The primary objectives of Installation Qualification include verification of installation, documentation review, and establishing baseline measurements for future validation phases.
Why is verification of installation important?
Verification of installation ensures that all components are installed according to the manufacturer's specifications and design requirements, which is essential for maintaining operational integrity.
What does the documentation review involve during Installation Qualification?
The documentation review involves a thorough examination of all necessary documentation, including installation manuals and specifications, to confirm accuracy and completeness, which is vital for regulatory adherence and future operations.
What are baseline measurements and why are they important?
Baseline measurements are performance metrics established during Installation Qualification that serve as a reference for comparing against future validation phases, facilitating the assessment of ongoing reliability and effectiveness of the equipment.
How often should requalification be conducted?
Requalification should be conducted regularly based on a risk evaluation and regulatory obligations to ensure continuous adherence to standards.
Can IQ, OQ, and PQ activities be consolidated?
Yes, for less complex systems, Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) activities can be consolidated into a single document, offering flexibility in documentation practices.
What is the significance of Installation Qualification beyond compliance?
The significance of Installation Qualification extends to being integral to the overall quality management system, enhancing production efficiency and ensuring consistent results in line with Good Manufacturing Practices (GMP).
What are the validation steps related to Installation Qualification?
The validation steps related to Installation Qualification include Installation Qualification (IQ), Installation Verification (IV), Operational Verification (OV), and Performance Verification (PV), which are essential for confirming that equipment and systems are installed and functioning correctly.
Why is Setup Verification important for the future of pharmaceutical compliance?
Setup Verification is important for the future of pharmaceutical compliance as organizations navigate increasingly intricate regulatory environments while ensuring product quality and safety, with its importance expected to remain paramount through 2025.
