Master Inspection Readiness: Essential Steps for Compliance Officers

Overview
The article emphasizes the critical steps compliance officers must undertake to achieve mastery in inspection readiness, highlighting its significance within the pharmaceutical and life sciences sectors. It delineates effective strategies for:
- Preparing documentation
- Managing the inspection process
- Implementing improvements post-inspection
These strategies are designed to ensure adherence to regulatory standards while cultivating a culture of continuous improvement within organizations, ultimately reinforcing the imperative of compliance as a cornerstone of operational excellence.
Introduction
In the highly regulated landscape of pharmaceuticals and life sciences, inspection readiness emerges as a critical pillar for compliance officers dedicated to meeting the stringent demands of authorities such as the FDA and EMA. Organizations that master this concept not only mitigate the risks of non-compliance but also foster trust with regulators and stakeholders, ultimately paving the way for long-term success. However, the journey toward achieving inspection readiness is fraught with challenges, ranging from ensuring meticulous documentation to effectively managing the inspection process itself.
What essential steps can compliance officers undertake to transform their organizations into models of preparedness and resilience?
Understand the Importance of Inspection Readiness
Inspection readiness is a pivotal element of compliance in the pharmaceutical and life sciences sectors, especially for entities aiming to meet the rigorous standards established by governing bodies like the FDA and EMA.
AVS Life Sciences offers comprehensive GxP compliance services, including GMP audits focused on APIs, drug products, and testing facilities, ensuring that companies are thoroughly prepared for unexpected evaluations.
The importance of inspection readiness for such evaluations lies in its ability to mitigate risks associated with non-compliance, which can lead to severe penalties, product recalls, or even the closure of facilities.
By fostering a culture of inspection readiness and leveraging the expertise of AVS Life Sciences, organizations can ensure that their processes, documentation, and quality systems consistently align with regulatory expectations.
This proactive strategy not only but also cultivates trust with regulators and stakeholders, ultimately contributing to the long-term success of the organization.

Prepare Your Documentation and Team for Success
To achieve , start by confirming that all documentation is precise, complete, and readily accessible. This encompasses standard operating procedures (SOPs), training records, and quality control documents. Conduct a thorough review of these materials to pinpoint any gaps or inconsistencies. Additionally, it is crucial to educate your team on evaluation protocols and expectations. Implement practice evaluations to simulate the actual experience, allowing team members to rehearse their responses and familiarize themselves with the documentation. Assign specific roles to team members during the evaluation to ensure a coordinated and efficient response.
Furthermore, incorporating a comprehensive checklist for [Computer System Validation (CSV)](https://avslifesciences.com/job-opportunities/computer-system-validation-csv) can significantly enhance your preparation. This checklist should address all stages of CSV, including:
- Planning
- Defining User Requirement Specifications (URS)
- Design specifications
- Various qualification testing phases (IQ, OQ, PQ)
By ensuring that your CSV processes are robust and well-documented, you not only bolster confidence but also enhance inspection readiness and the overall effectiveness of the evaluation process, aligning with FDA regulations and quality management practices.
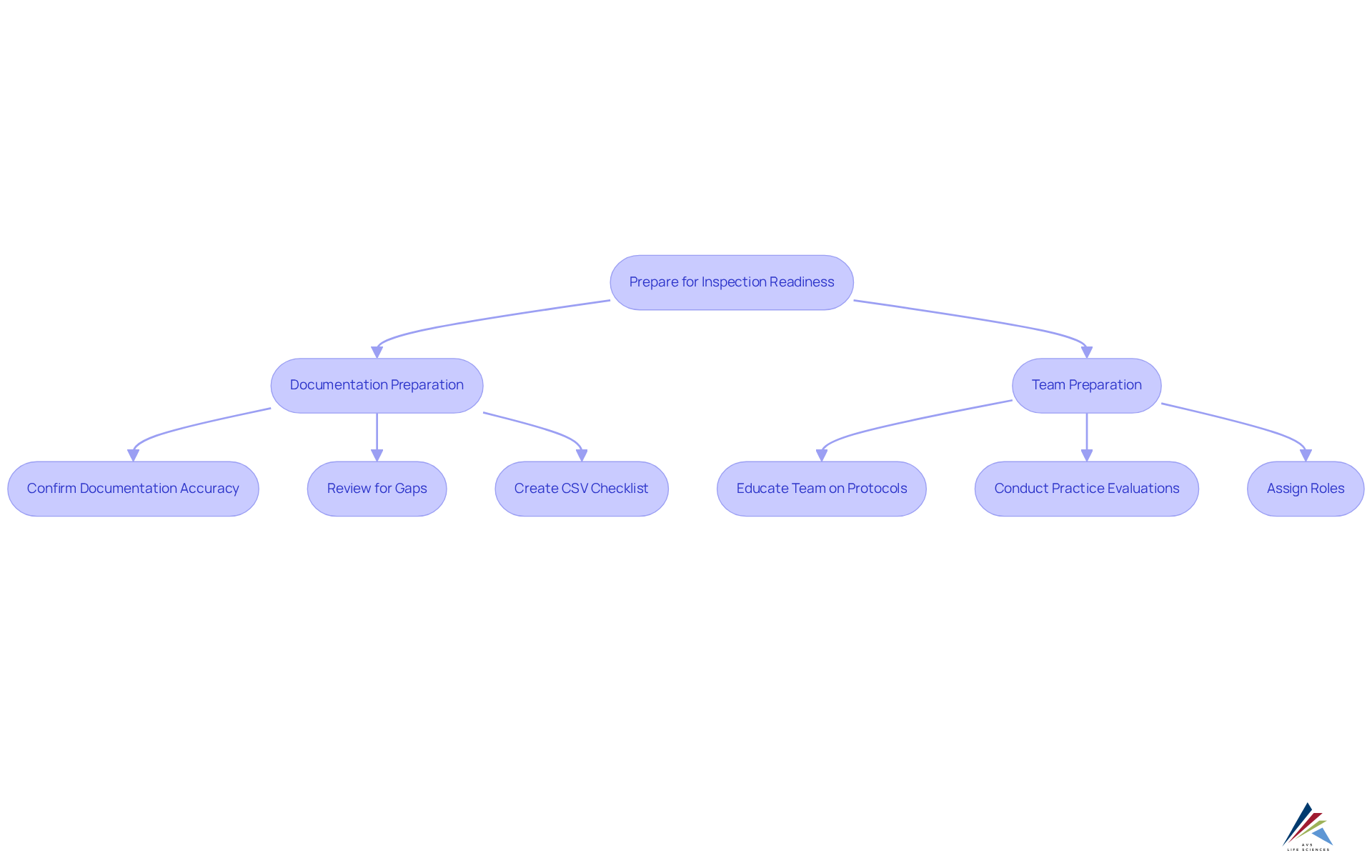
Manage the Inspection Process Effectively
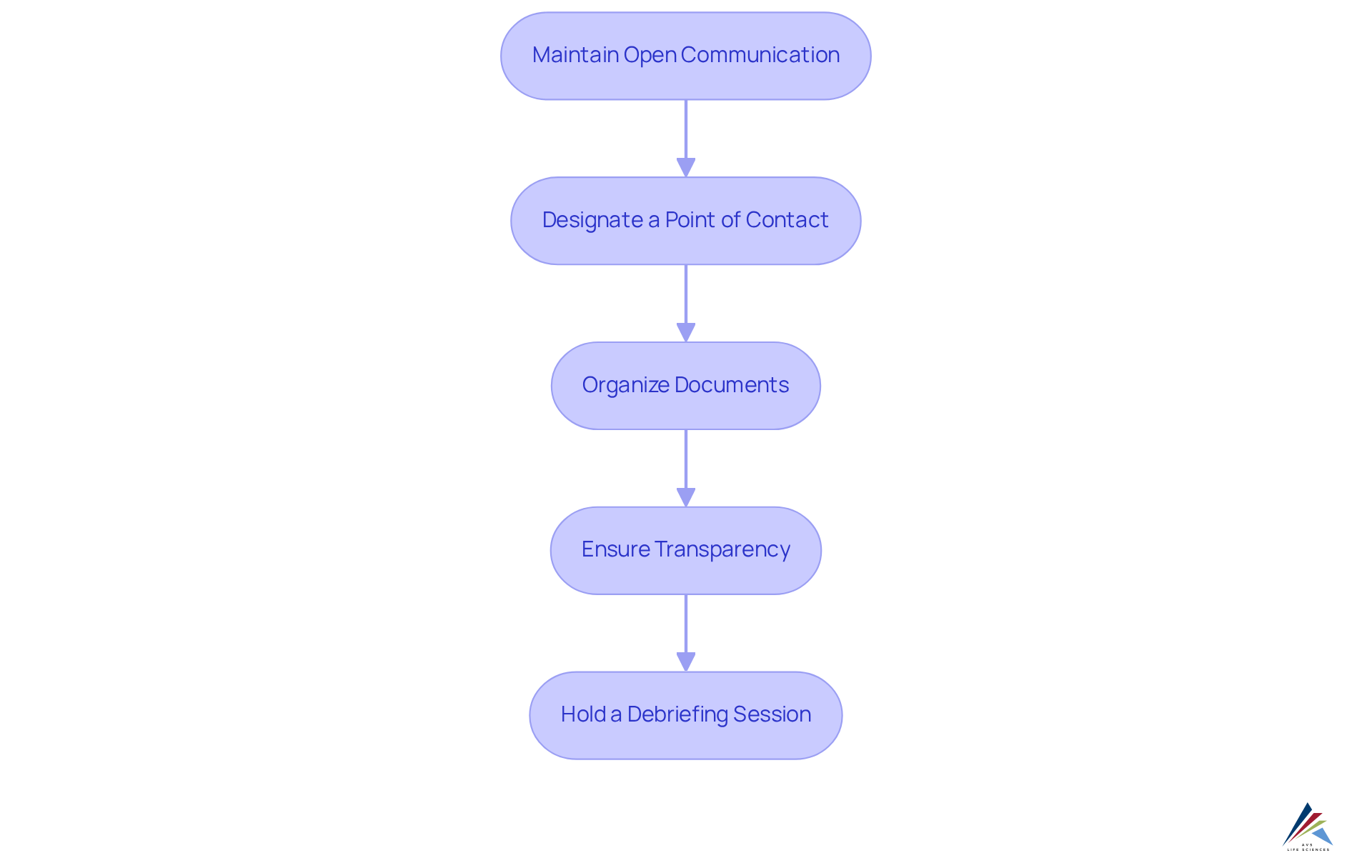
During the evaluation, it is imperative to maintain open communication with the inspectors. Designate a primary point of contact capable of facilitating the flow of information and addressing any questions or concerns that may arise. Ensure that all requested documents are readily available and meticulously organized to achieve inspection readiness and minimize delays. in your responses are crucial; if you do not know the answer to a question, it is preferable to admit it rather than provide incorrect information. Following the review, hold a debriefing session with your team to discuss the findings and identify areas for enhancement. This collaborative approach not only addresses immediate concerns but also fosters a culture of continuous improvement within the organization.
Implement Post-Inspection Improvements and Learnings
Following the inspection, it is imperative to review the findings and formulate a corrective action plan to address any identified issues. Engaging your team in this process is crucial to ensure that everyone understands the necessary changes and their respective responsibilities in implementing them. This becomes especially significant in the realm of GMP audits, where , drug products, and testing facilities is of utmost importance.
Monitor the progress of these enhancements and establish criteria to evaluate their effectiveness, particularly in relation to the comprehensive phases of the Computer System Validation (CSV) framework. Additionally, consider conducting regular training sessions to reinforce compliance practices and keep your team informed about any regulatory changes.
By cultivating a culture of continuous improvement and learning, your organization can significantly enhance its inspection readiness while also improving its overall compliance posture, ensuring that all systems and processes align with the stringent standards set forth by regulatory bodies.

Conclusion
Inspection readiness is a cornerstone for compliance officers in the pharmaceutical and life sciences industries, highlighting the imperative of being prepared for evaluations from regulatory bodies such as the FDA and EMA. By prioritizing inspection readiness, organizations not only protect themselves from potential compliance pitfalls but also cultivate trust with stakeholders, ultimately driving long-term success.
This article delineates essential steps for achieving inspection readiness, including:
- Meticulous preparation of documentation
- Effective team training
- Proactive management of the inspection process
Key strategies, such as maintaining open communication with inspectors, conducting practice evaluations, and implementing corrective action plans post-inspection, are crucial for ensuring that organizations can meet regulatory standards and continuously enhance their compliance posture.
In an environment where regulatory scrutiny is intensifying, the commitment to inspection readiness is paramount. Embracing a culture of continuous improvement and learning empowers organizations to navigate inspections successfully while enhancing their overall operational integrity. By implementing these strategies, compliance officers can profoundly influence their organization’s capacity to thrive in a highly regulated landscape, ensuring they remain prepared for the challenges that lie ahead.
Frequently Asked Questions
What is inspection readiness in the pharmaceutical and life sciences sectors?
Inspection readiness refers to the preparedness of organizations to meet the compliance standards set by regulatory bodies like the FDA and EMA, ensuring they are ready for evaluations and inspections.
Why is inspection readiness important?
It is crucial for mitigating risks associated with non-compliance, which can result in severe penalties, product recalls, or facility closures.
What services does AVS Life Sciences provide related to inspection readiness?
AVS Life Sciences offers comprehensive GxP compliance services, including GMP audits focused on APIs, drug products, and testing facilities, to help organizations prepare for unexpected evaluations.
How can organizations foster a culture of inspection readiness?
Organizations can foster a culture of inspection readiness by ensuring that their processes, documentation, and quality systems consistently align with regulatory expectations and by leveraging the expertise of compliance service providers like AVS Life Sciences.
What are the benefits of being inspection ready?
Being inspection ready enhances compliance, cultivates trust with regulators and stakeholders, and contributes to the long-term success of the organization.
