Master ICH Q2 R2 for Effective Pharmaceutical Compliance

Overview
This article addresses the mastery of ICH Q2 R2 guidelines, a crucial aspect for achieving effective compliance within the pharmaceutical industry. It underscores the significance of validating analytical procedures through a comprehensive approach. This approach encompasses:
- Defining analytical targets
- Selecting appropriate validation parameters
- Implementing continuous training
Collectively, these elements enhance the reliability and compliance of analytical methods, ensuring that organizations meet the stringent requirements of the industry.
Introduction
Navigating the complexities of pharmaceutical compliance often feels like traversing a labyrinth, particularly when adhering to critical guidelines such as ICH Q2 R2. This pivotal framework outlines the validation of analytical procedures and plays a crucial role in ensuring that these methods are reliable and fit for their intended purposes. As the pharmaceutical landscape evolves, organizations face the pressing challenge of maintaining compliance while striving for continuous improvement. How can they effectively implement ICH Q2 R2 to enhance their analytical practices and overcome the obstacles that lie ahead?
Clarify the Fundamentals of ICH Q2 R2
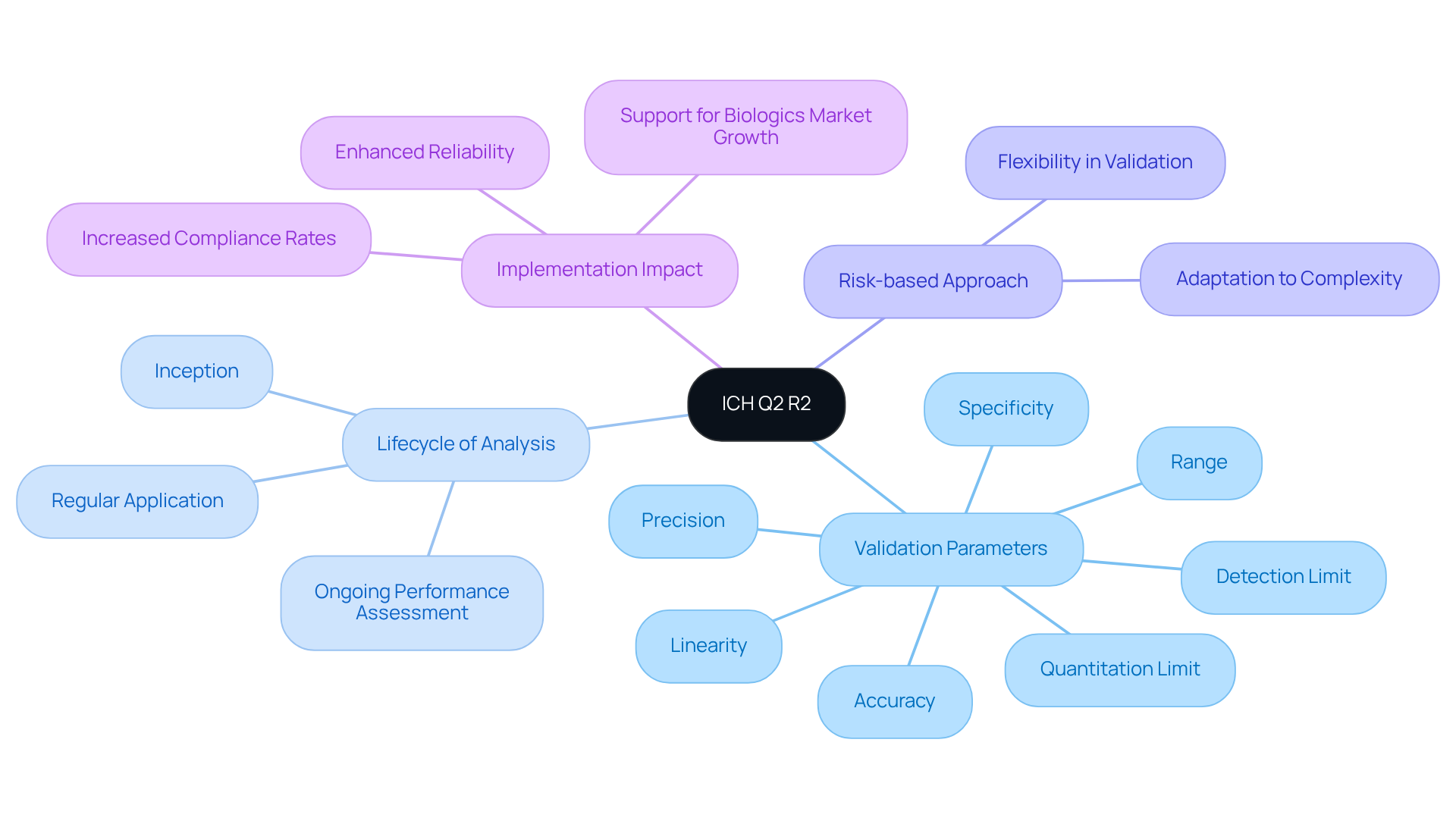
ICH Q2 R2 is a pivotal guideline established by the International Council for Harmonisation (ICH), which delineates the validation of analytical procedures. Its emphasis on ensuring that analytical techniques are fit for their intended purpose is paramount for maintaining regulatory compliance within the pharmaceutical industry, particularly in relation to ich q2 r2. The key components of ICH Q2 R2 encompass:
- Validation Parameters: Critical parameters such as accuracy, precision, specificity, detection limit, quantitation limit, linearity, and range must undergo rigorous evaluation to ascertain that the analytical method meets its intended application.
- Lifecycle of Analysis: This guideline underscores the importance of overseeing the entire lifecycle of an analytical procedure, including ich q2 r2, from its inception to its regular application and ongoing performance assessment. This comprehensive perspective is vital for ensuring adherence and quality assurance.
The ICH Q2 R2 document advocates for a risk-based approach, promoting a risk-oriented validation strategy that allows flexibility in the validation process based on the complexity and significance of the analytical technique. This approach not only enhances success rates in compliance but also adapts to the evolving landscape of the pharmaceutical industry.
The implementation of ich q2 r2 is expected to significantly enhance the reliability of analytical methods, thereby increasing compliance rates across the industry. With the biologics market projected to expand at a until 2027, the significance of these guidelines becomes increasingly evident. Real-world examples of effective lifecycle management serve to illustrate the practical application of these principles, demonstrating how organizations can adeptly navigate the intricacies of compliance requirements while upholding high standards of quality and efficacy.
Implement ICH Q2 R2 in Analytical Procedures
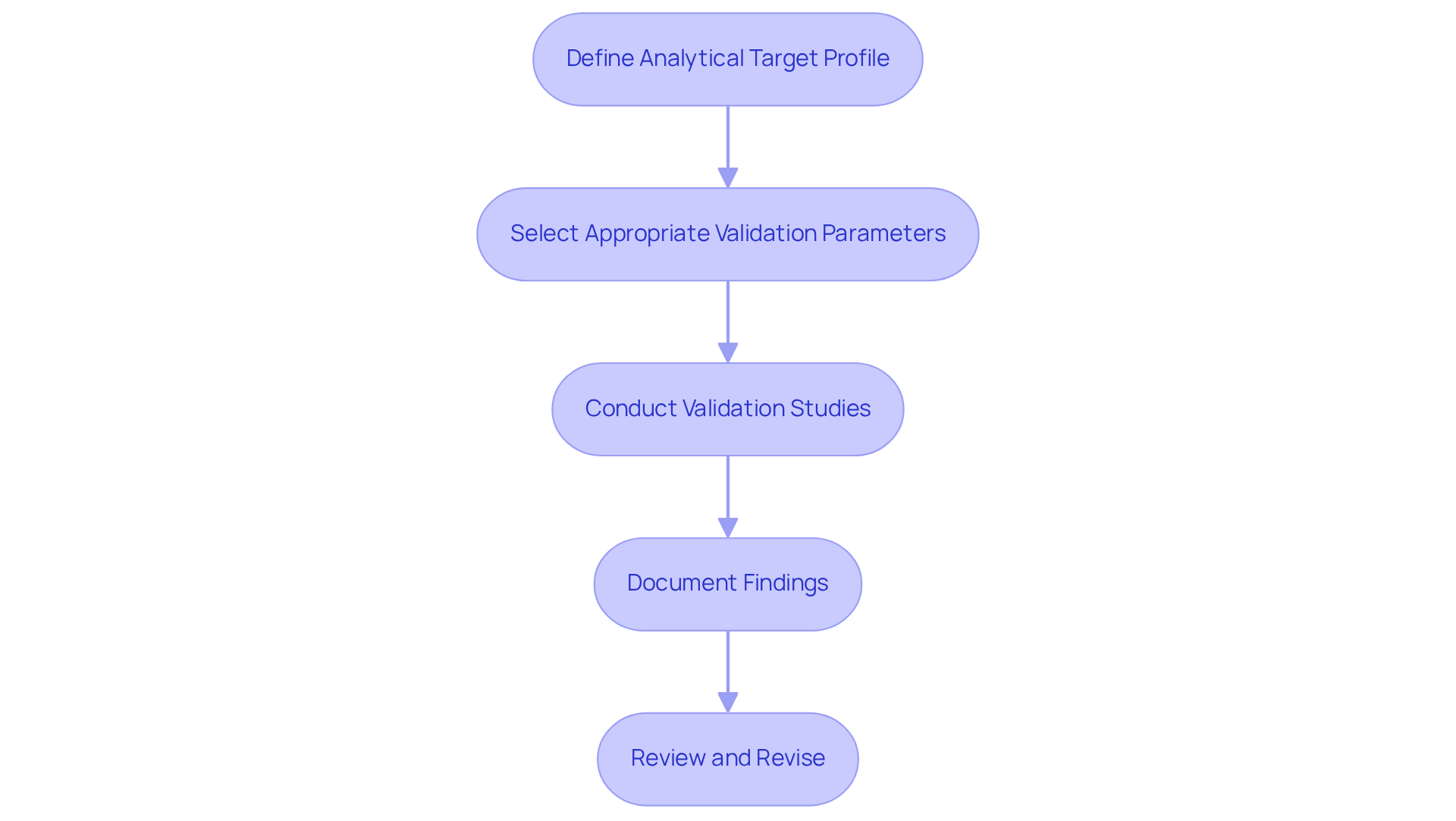
To effectively implement ICH Q2 R2 in analytical procedures, it is essential to adhere to the following best practices:
- Define the Analytical Target Profile (ATP): Clearly outline the expected characteristics of the analytical approach, including its intended purpose and the specific quality attributes it must measure. This foundational step ensures alignment with and project requirements.
- Select Appropriate Validation Parameters: Identify the validation parameters relevant to the technique's intended use. For instance, if the approach is intended for potency testing, prioritize parameters such as accuracy, precision, and specificity to ensure reliable results.
- Conduct Validation Studies: Execute experiments to evaluate each selected parameter. A linearity study, for instance, can be conducted to assess the technique's performance across a range of concentrations, confirming its reliability and robustness. Furthermore, consider the permissible signal-to-noise ratios of 3:1 for detection and 10:1 for quantitation, which are essential for confirming analytical procedures.
- Document Findings: Maintain comprehensive documentation of all validation studies, detailing methodologies, results, and conclusions. This documentation is essential for regulatory submissions and audits, serving as proof of adherence and procedure reliability.
- Review and Revise: Regularly evaluate the analytical approach and validation data to ensure ongoing compliance. Make necessary adjustments based on new information, technological advancements, or changes in regulations to maintain the approach's effectiveness and relevance. Integrating systematic process development and risk-based strategies, as highlighted in the ICH Q2 R2 guidelines, can further enhance the justification behind these steps.
A transformative case study exemplifies these principles: AVS Life Sciences assisted a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility. This project was completed on time and within budget, showcasing AVS's dedication to quality assurance and adherence to standards. Throughout this process, AVS ensured that each step adhered to stringent quality control measures, including defining the ATP and selecting appropriate validation parameters. The collaboration allowed the client to focus on developing medicines while AVS managed the complexities of ICH Q2 R2. Such real-life applications underscore the importance of following these steps, illustrating how organizations can effectively navigate the complexities of ICH Q2 R2. By adhering to these best practices, pharmaceutical professionals can enhance the reliability and compliance of their analytical methods, ultimately facilitating successful regulatory outcomes.
Navigate Compliance Challenges with ICH Q2 R2
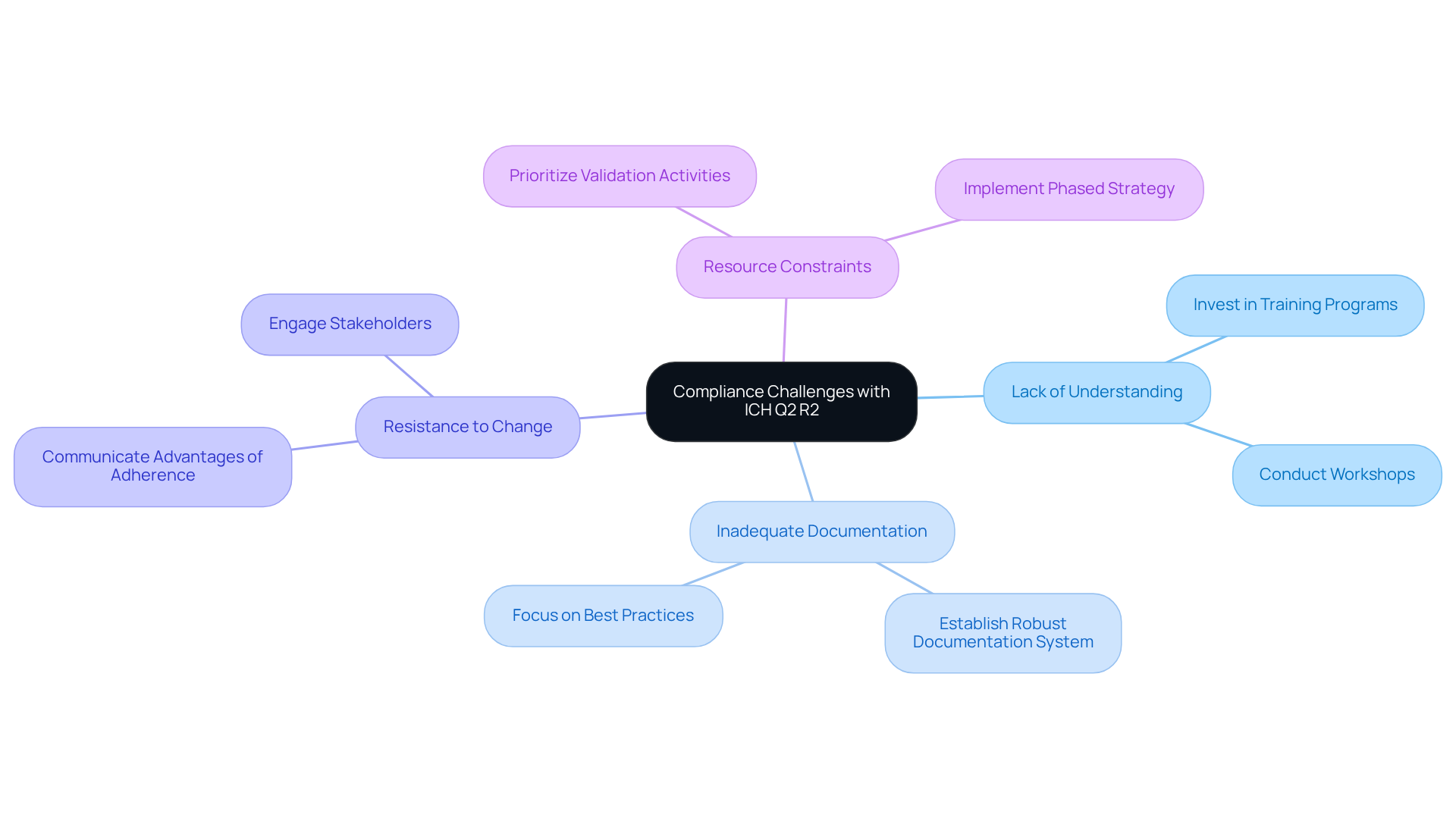
Compliance with ich q2 r2 presents significant challenges that organizations must navigate effectively. Key issues and strategies to address them include the following:
- Lack of Understanding: The complexities of ICH Q2 R2 can be daunting. Organizations should invest in comprehensive training programs tailored to the guidelines. Workshops covering the on analytical procedures can significantly enhance staff knowledge and confidence. AVS Life Sciences offers specialized training sessions designed to demystify these complexities and empower teams.
- Inadequate Documentation: Insufficient documentation frequently results in regulatory failures. Establishing a robust documentation system is crucial. This system must meticulously capture all validation activities and results, ensuring that records are readily accessible for audits. Training programs focusing on best practices in documentation can further reinforce this aspect. AVS Life Sciences offers consulting services to help organizations create effective documentation strategies that are in accordance with ich q2 r2 requirements.
- Resistance to Change: Transitioning to ICH Q2 R2 often necessitates changes in established practices, which can meet resistance. To promote a culture of ongoing enhancement, it is vital to clearly convey the advantages of adherence. Engaging stakeholders through regular updates and success stories can help build support for necessary changes. For example, a pharmaceutical company successfully implemented ich q2 r2 with the guidance of AVS Life Sciences, and shared case studies of improved outcomes with their teams.
- Resource Constraints: Limited resources can hinder adherence efforts. Organizations should prioritize validation activities based on risk assessments, ensuring that critical methods receive validation first. Implementing a phased strategy for adherence can help manage resource allocation effectively. AVS Life Sciences provides customized solutions to assist organizations in optimizing their resources and simplifying regulatory processes.
By addressing these challenges with focused strategies and leveraging the expertise of AVS Life Sciences, organizations can enhance their adherence to ich q2 r2, which will ultimately result in improved quality and oversight outcomes.
Embrace Continuous Improvement in Compliance Practices
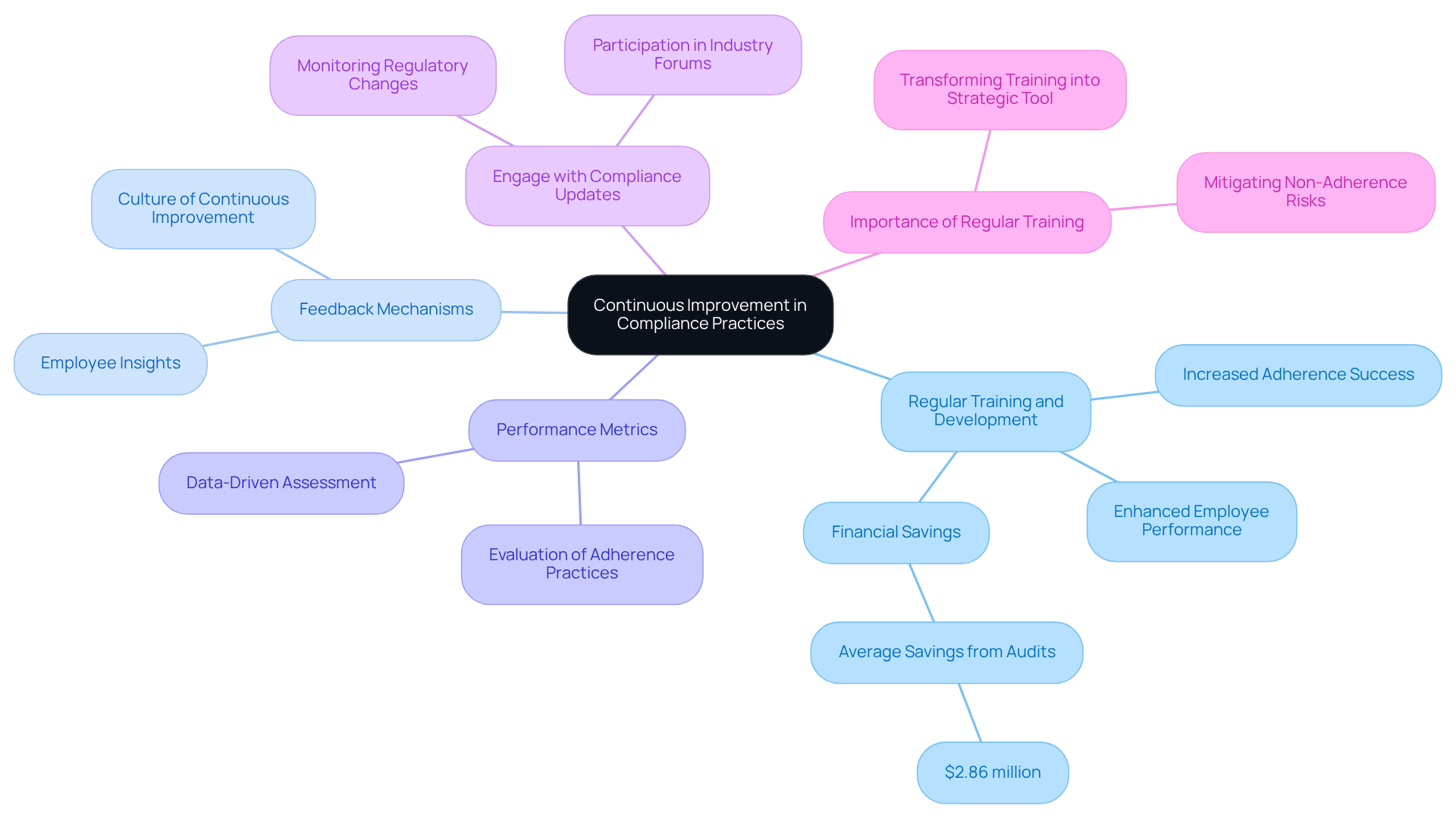
To foster continuous improvement in compliance practices, organizations must adopt effective strategies:
- Regular Training and Development: Ongoing training is essential for keeping staff updated on the latest regulatory changes and best practices in analytical method validation. This commitment guarantees that teams are well-informed and prepared to adapt to evolving regulatory environments. Statistics indicate that organizations emphasizing regular training experience a notable increase in adherence success, enhanced employee performance, and diminished legal risks. For instance, companies saved an average of $2.86 million through regular audits, underscoring the financial advantages of ongoing education. AVS Life Sciences offers expert training programs tailored to the specific needs of the pharmaceutical and biotech industries, ensuring that staff are well-prepared to meet regulatory demands.
- Feedback Mechanisms: Establishing robust feedback loops enables employees to express challenges and suggest improvements to regulatory processes. Employee feedback provides insights into training quality and relevance, empowering staff and fostering a culture of continuous improvement and accountability. AVS Life Sciences encourages organizations to implement these mechanisms as part of their comprehensive quality management strategies.
- Performance Metrics: Implementing specific metrics to evaluate the effectiveness of adherence practices is crucial. Regular reviews of these metrics help identify areas needing improvement and track progress over time. For instance, organizations employing data-driven methods to assess training effectiveness can significantly enhance their regulatory initiatives. The typical duration to identify a data breach is 201 days, highlighting the importance of monitoring adherence effectiveness. AVS Life Sciences supports clients in creating and tracking these metrics to ensure continuous adherence and quality assurance.
- Engage with Compliance Updates: Staying informed about changes in regulations, such as ich q2 r2 updates, is vital. Engaging in industry forums and discussions allows organizations to exchange insights and learn from colleagues, promoting a collaborative atmosphere that improves regulatory strategies. Notably, 27% of Chief Compliance Officers (CCOs) strongly concur that they monitor and track changes in regulations, emphasizing the importance of active participation in adherence activities. AVS Life Sciences provides resources and support to help organizations stay abreast of these changes.
- Importance of Regular Training: Consistent training on policy updates is not merely advantageous; it is crucial for upholding standards. Entities allocating resources for ongoing training of their staff are better equipped to manage intricate compliance landscapes and mitigate risks linked to non-adherence. A data-driven approach to regulatory training transforms it from a checkbox task to a strategic instrument for business success. AVS Life Sciences' comprehensive GXP regulatory services, including GMP audits, ensure that organizations possess the knowledge and skills necessary to excel in compliance.
Conclusion
Mastering ICH Q2 R2 is essential for ensuring that pharmaceutical companies maintain compliance with regulatory standards while enhancing the reliability of their analytical methods. This guideline emphasizes the importance of validating analytical procedures and encourages a risk-based approach to validation, enabling organizations to adapt to the ever-evolving pharmaceutical landscape.
Key aspects of ICH Q2 R2 have been explored, including:
- The critical validation parameters that require rigorous evaluation
- The lifecycle of analysis
- Best practices for effective implementation
The challenges organizations face in adhering to these guidelines, such as inadequate documentation and resistance to change, have been addressed, along with strategies to overcome these obstacles. Real-world examples demonstrate how companies can successfully navigate compliance requirements while maintaining high standards of quality.
Ultimately, embracing ICH Q2 R2 fosters regulatory compliance and promotes a culture of continuous improvement within organizations. By investing in training, establishing feedback mechanisms, and actively engaging with compliance updates, pharmaceutical professionals can enhance their analytical practices and ensure sustained success in meeting regulatory demands. The importance of ICH Q2 R2 in the pharmaceutical industry cannot be overstated; it serves as a foundational element for quality assurance and operational excellence.
Frequently Asked Questions
What is ICH Q2 R2?
ICH Q2 R2 is a guideline established by the International Council for Harmonisation (ICH) that focuses on the validation of analytical procedures in the pharmaceutical industry.
Why is ICH Q2 R2 important?
It is important because it ensures that analytical techniques are fit for their intended purpose, which is crucial for maintaining regulatory compliance within the pharmaceutical industry.
What are the key components of ICH Q2 R2?
The key components include validation parameters such as accuracy, precision, specificity, detection limit, quantitation limit, linearity, and range, as well as the lifecycle of analysis and a risk-based approach to validation.
What validation parameters are evaluated under ICH Q2 R2?
The validation parameters evaluated include accuracy, precision, specificity, detection limit, quantitation limit, linearity, and range.
What does the lifecycle of analysis refer to in ICH Q2 R2?
The lifecycle of analysis refers to overseeing the entire process of an analytical procedure from its inception to its regular application and ongoing performance assessment.
What is the risk-based approach advocated by ICH Q2 R2?
The risk-based approach promotes a risk-oriented validation strategy that allows flexibility in the validation process based on the complexity and significance of the analytical technique.
How does ICH Q2 R2 enhance compliance in the pharmaceutical industry?
By implementing ICH Q2 R2, organizations can enhance the reliability of analytical methods, leading to increased compliance rates across the industry.
What is the projected growth of the biologics market, and why is it relevant to ICH Q2 R2?
The biologics market is projected to expand at a compound annual growth rate of 15% until 2027, highlighting the increasing significance of ICH Q2 R2 guidelines in ensuring compliance and quality in a growing industry.
Are there real-world examples of lifecycle management related to ICH Q2 R2?
Yes, real-world examples illustrate the practical application of ICH Q2 R2 principles, showing how organizations navigate compliance requirements while maintaining high standards of quality and efficacy.
