Master FDA Labeling Requirements for Skincare and OTC Products

Overview
This article delves into the critical FDA labeling requirements for skincare and over-the-counter (OTC) products, underscoring the necessity of compliance to avert legal complications. It articulates essential regulations, including:
- Ingredient declaration
- Truthful labeling claims
- Differentiation between OTC and cosmetic products
Furthermore, it offers manufacturers strategic insights to navigate these intricate requirements effectively, thereby bolstering consumer trust and ensuring adherence to regulatory standards.
Introduction
Navigating the intricate landscape of FDA labeling requirements for skincare and over-the-counter (OTC) products is essential for manufacturers striving to maintain compliance and build consumer trust. This tutorial delves into the critical aspects of these regulations, offering insights into:
- Ingredient declarations
- Labeling claims
- Distinctions between OTC and cosmetic products
As the regulatory environment continues to evolve, companies must ensure they not only meet current standards but also stay ahead of potential changes that could impact their operations. By understanding these challenges and implementing effective compliance strategies, manufacturers can enhance their credibility and foster consumer confidence.
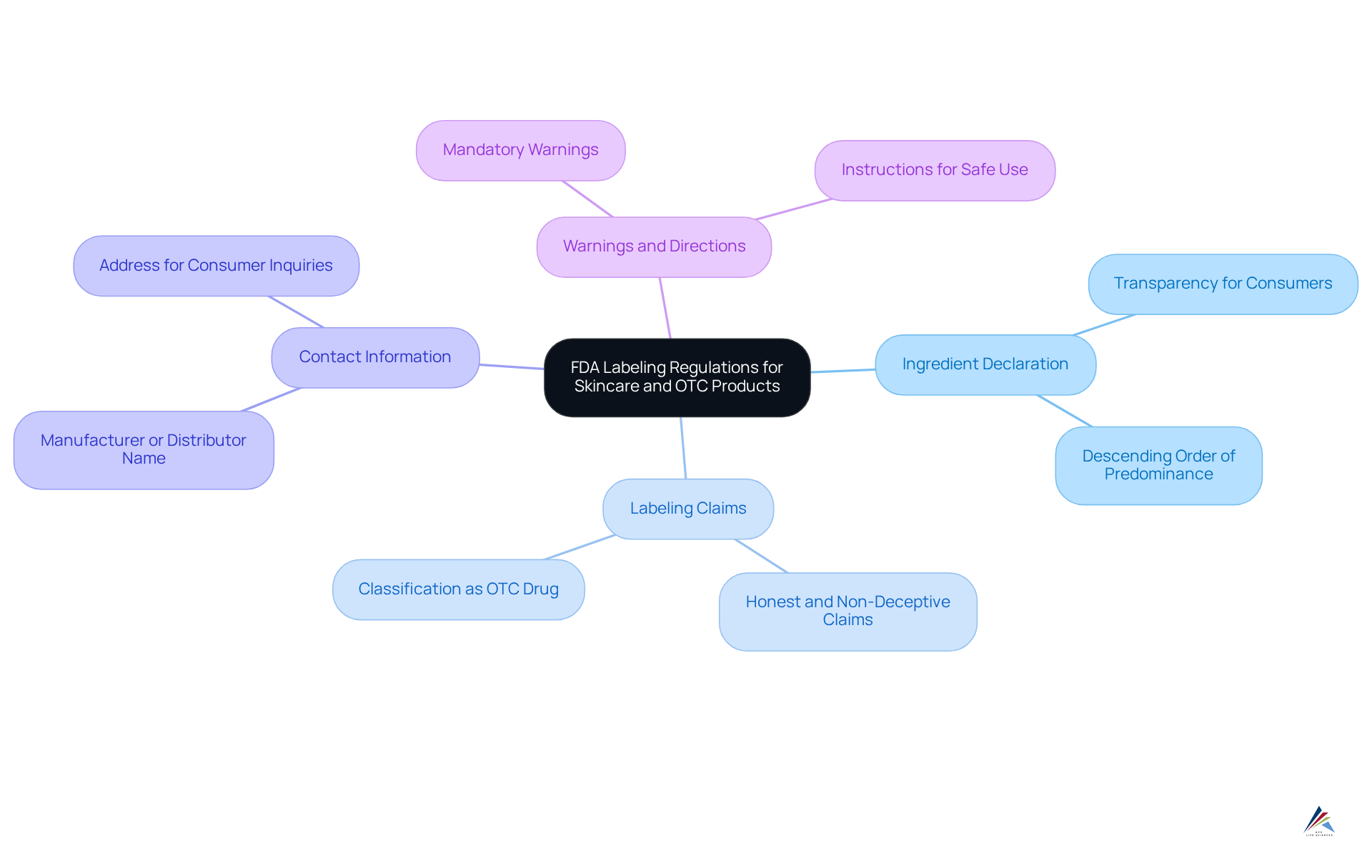
Clarify FDA Labeling Regulations for Skincare and OTC Products
The FDA is responsible for overseeing the FDA labeling requirements for skincare and OTC products in accordance with the Federal Food, Drug, and Cosmetic Act (FDCA). Understanding the for skincare and OTC products is crucial for ensuring compliance and avoiding potential legal issues. These regulations include:
- Ingredient Declaration: All ingredients must be listed in descending order of predominance on the product label, ensuring transparency for consumers.
- Labeling Claims: Any assertions made about the product must be honest and not deceptive. For instance, if a product claims to treat a skin condition, it may be classified as an OTC drug, necessitating adherence to stricter regulations.
- Contact Information: The label must prominently display the name and address of the manufacturer or distributor, facilitating consumer inquiries or complaints.
- Warnings and Directions: Specific warnings and instructions for use are mandatory, particularly for products that may pose risks if misused.
By understanding the FDA labeling requirements for skincare and OTC products, companies can effectively navigate the complexities of compliance, thereby safeguarding their operations and enhancing consumer trust.
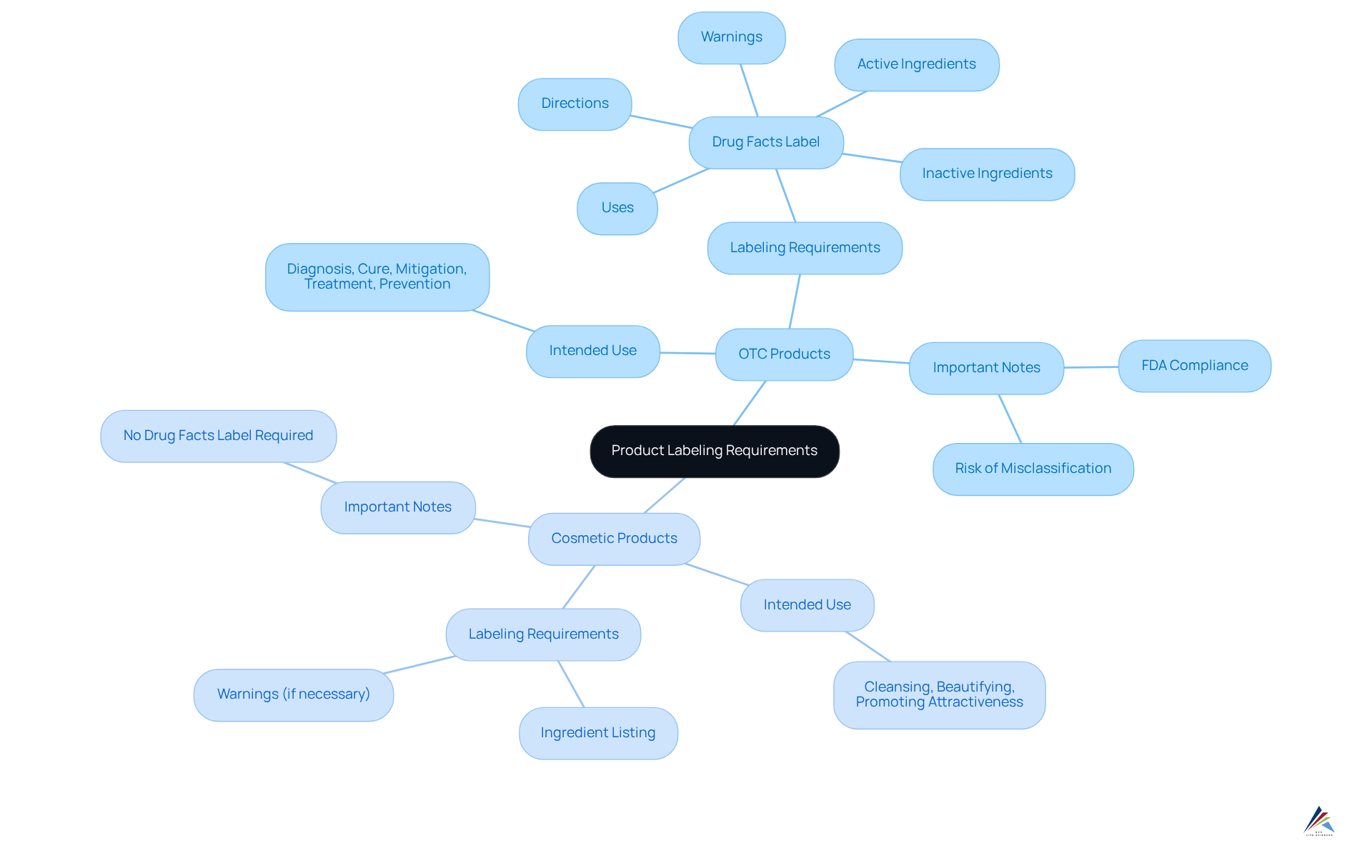
Differentiate Between OTC and Cosmetic Product Labeling Requirements
The distinction between OTC and cosmetic products is fundamentally rooted in their intended use and claims.
OTC Products are specifically designed for the diagnosis, cure, mitigation, treatment, or prevention of disease. These products must adhere to the for skincare and OTC products, which include a 'Drug Facts' label that details active ingredients, uses, warnings, directions, and inactive ingredients.
In contrast, Cosmetic Products focus on cleansing, beautifying, promoting attractiveness, or altering appearance without influencing the body's structure or functions. While they are not required to display a 'Drug Facts' label, they must list ingredients and include any necessary warnings.
Understanding these differences is crucial for manufacturers. Accurate labeling that adheres to FDA labeling requirements for skincare and OTC products prevents misclassification and mitigates the risk of regulatory penalties, ensuring compliance in a complex landscape.
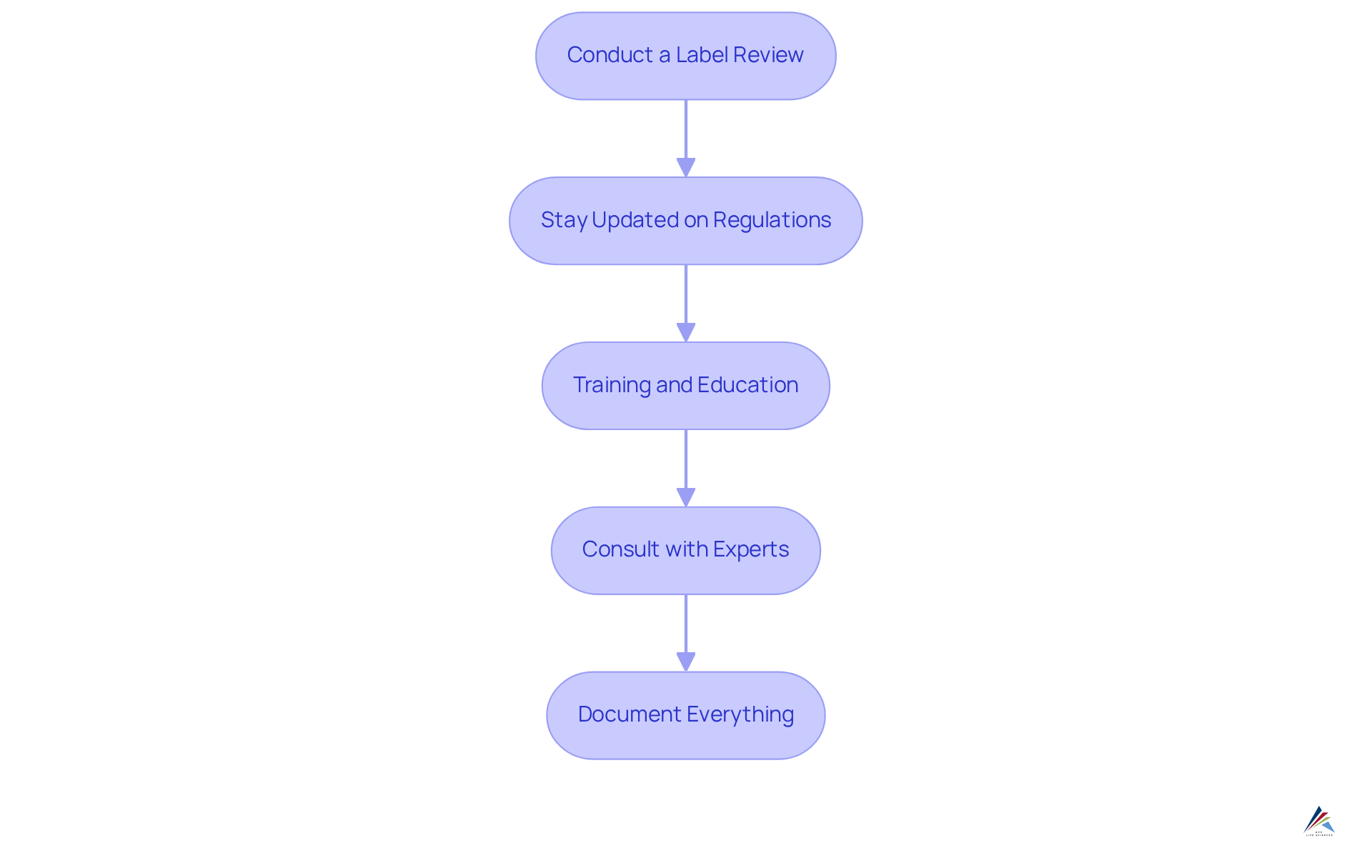
Implement Effective Strategies for Compliance with FDA Labeling Standards
To ensure compliance with FDA labeling standards, manufacturers must adopt effective strategies that address the challenges of regulatory adherence:
- Conduct a Label Review: Regularly examine labels against FDA requirements to ensure all necessary information is included and correct. This proactive approach mitigates potential compliance issues.
- Stay Updated on Regulations: It is crucial to keep abreast of changes in FDA regulations, particularly the FDA labeling requirements for skincare and OTC products, including those associated with the Modernization of Cosmetics Regulation Act (MoCRA), which may affect labeling requirements. Being informed allows for timely adjustments to labeling practices.
- Training and Education: Ongoing training for staff involved in product development and labeling is essential. Ensuring that team members understand regulatory requirements fosters a culture of compliance.
- Consult with Experts: Engaging regulatory specialists to review labeling practices provides invaluable insights and advice on best methods. Their expertise can guide manufacturers in navigating complex regulations.
- Document Everything: Keeping detailed records of labeling choices and modifications is vital. This documentation showcases adherence during audits and reinforces the commitment to compliance.
By implementing these strategies, manufacturers can significantly reduce the risk of non-compliance and enhance their market readiness, positioning themselves as leaders in the industry.
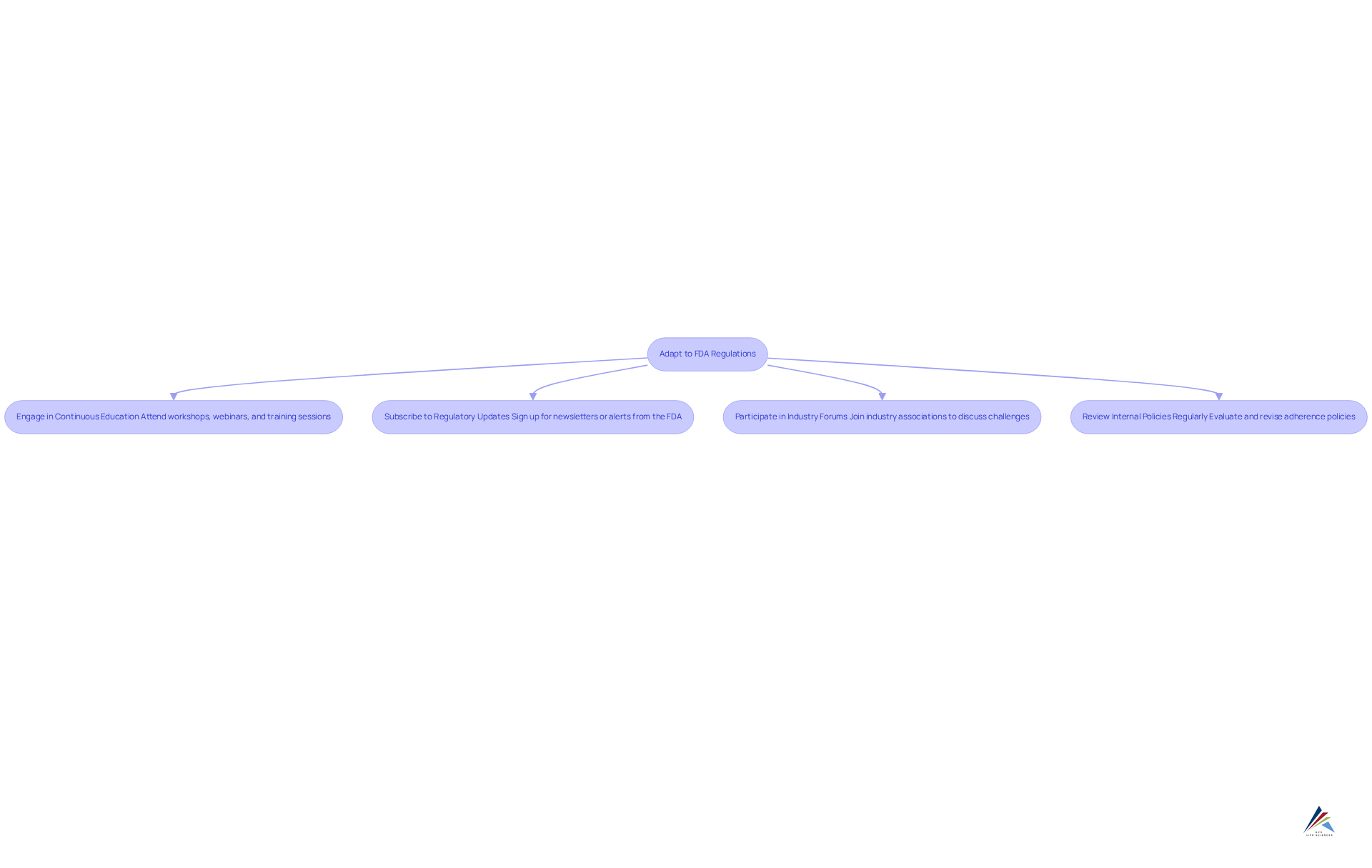
Adapt to Evolving FDA Regulations: Continuous Learning and Compliance
The regulatory landscape is constantly evolving for skincare and OTC products, posing significant compliance challenges for businesses in relation to FDA labeling requirements for skincare and OTC products. To navigate this complexity and ensure adherence to regulations, companies must take proactive measures:
- Engage in Continuous Education: Attend workshops, webinars, and training sessions focused on FDA regulations and compliance strategies to stay informed.
- Subscribe to Regulatory Updates: Sign up for newsletters or alerts from the FDA and industry organizations to receive timely updates on regulatory changes that could impact operations.
- Participate in Industry Forums: Join industry associations or forums where professionals discuss regulatory challenges and share best practices, fostering a collaborative approach to compliance.
- Review Internal Policies Regularly: Frequently evaluate and revise internal adherence policies to align with the most recent rules and industry standards, ensuring that your organization remains compliant.
By fostering a culture of continuous learning and adaptation, companies can better navigate the complexities of the FDA labeling requirements for skincare and OTC products to maintain compliance. This commitment not only mitigates risks but also positions organizations as leaders in the industry, capable of responding effectively to regulatory changes.
Conclusion
Understanding the intricacies of FDA labeling requirements for skincare and OTC products is essential for manufacturers aiming to ensure compliance and protect their business interests. By grasping these regulations, companies can not only avoid legal pitfalls but also foster consumer trust through transparency and accountability in their labeling practices.
The article highlights several critical aspects of FDA labeling regulations, including:
- The necessity for ingredient declaration
- Honest labeling claims
- The provision of clear warnings and directions
It also emphasizes the distinctions between OTC and cosmetic products, detailing how each category necessitates different labeling approaches. Furthermore, implementing effective compliance strategies, such as regular label reviews and continuous education, is crucial for navigating the ever-evolving regulatory landscape.
In a rapidly changing environment, companies must prioritize ongoing education and remain vigilant about updates in FDA regulations. By embracing a proactive stance towards compliance, businesses can not only mitigate risks associated with non-compliance but also position themselves as industry leaders. The commitment to understanding and adapting to FDA labeling requirements is not just a regulatory obligation; it is a pathway to building a reputable brand that consumers can trust.
Frequently Asked Questions
What are the FDA's responsibilities regarding skincare and OTC product labeling?
The FDA oversees the labeling requirements for skincare and OTC products in accordance with the Federal Food, Drug, and Cosmetic Act (FDCA).
Why is it important to understand FDA labeling requirements for skincare and OTC products?
Understanding these requirements is crucial for ensuring compliance and avoiding potential legal issues.
What is included in the ingredient declaration for skincare products?
All ingredients must be listed in descending order of predominance on the product label to ensure transparency for consumers.
What are the regulations regarding labeling claims for skincare products?
Any claims made about the product must be honest and not deceptive. For example, if a product claims to treat a skin condition, it may be classified as an OTC drug, which requires adherence to stricter regulations.
What contact information must be included on the product label?
The label must prominently display the name and address of the manufacturer or distributor to facilitate consumer inquiries or complaints.
Are there specific requirements for warnings and directions on skincare product labels?
Yes, specific warnings and instructions for use are mandatory, especially for products that may pose risks if misused.
How can companies benefit from understanding FDA labeling requirements?
By understanding these requirements, companies can effectively navigate compliance complexities, safeguard their operations, and enhance consumer trust.
