Master FDA 21 CFR Part 11, EU Annex 11, and GAMP 5 Compliance

Overview
The article presents a comprehensive overview of mastering compliance with FDA 21 CFR Part 11, EU Annex 11, and GAMP 5, outlining essential requirements and strategies for effective implementation. It highlights the critical importance of:
- Rigorous validation
- Comprehensive audit trails
- Thorough employee training
Evidence supports the assertion that adherence to these frameworks significantly enhances data integrity and operational efficiency within the life sciences sector. By addressing compliance challenges head-on, the article not only builds interest but also generates a desire for robust compliance solutions, ultimately prompting action towards engagement with AVS Life Sciences.
Introduction
Navigating the intricate landscape of regulatory compliance in the life sciences sector presents significant challenges. A deep understanding of key frameworks such as:
- FDA 21 CFR Part 11
- EU Annex 11
- GAMP 5
is essential. These standards are not merely bureaucratic hurdles; they represent critical guidelines that ensure data integrity and operational efficiency in electronic environments.
As regulations evolve and changes loom on the horizon for 2025, organizations grapple with a pressing question: how can they effectively implement these compliance strategies without compromising productivity?
To master these frameworks and transform potential obstacles into opportunities for improvement, organizations must take decisive steps. Exploring successful compliance projects can provide valuable insights and illustrate the path forward. By engaging with AVS Life Sciences, organizations can navigate these complexities with confidence and expertise.
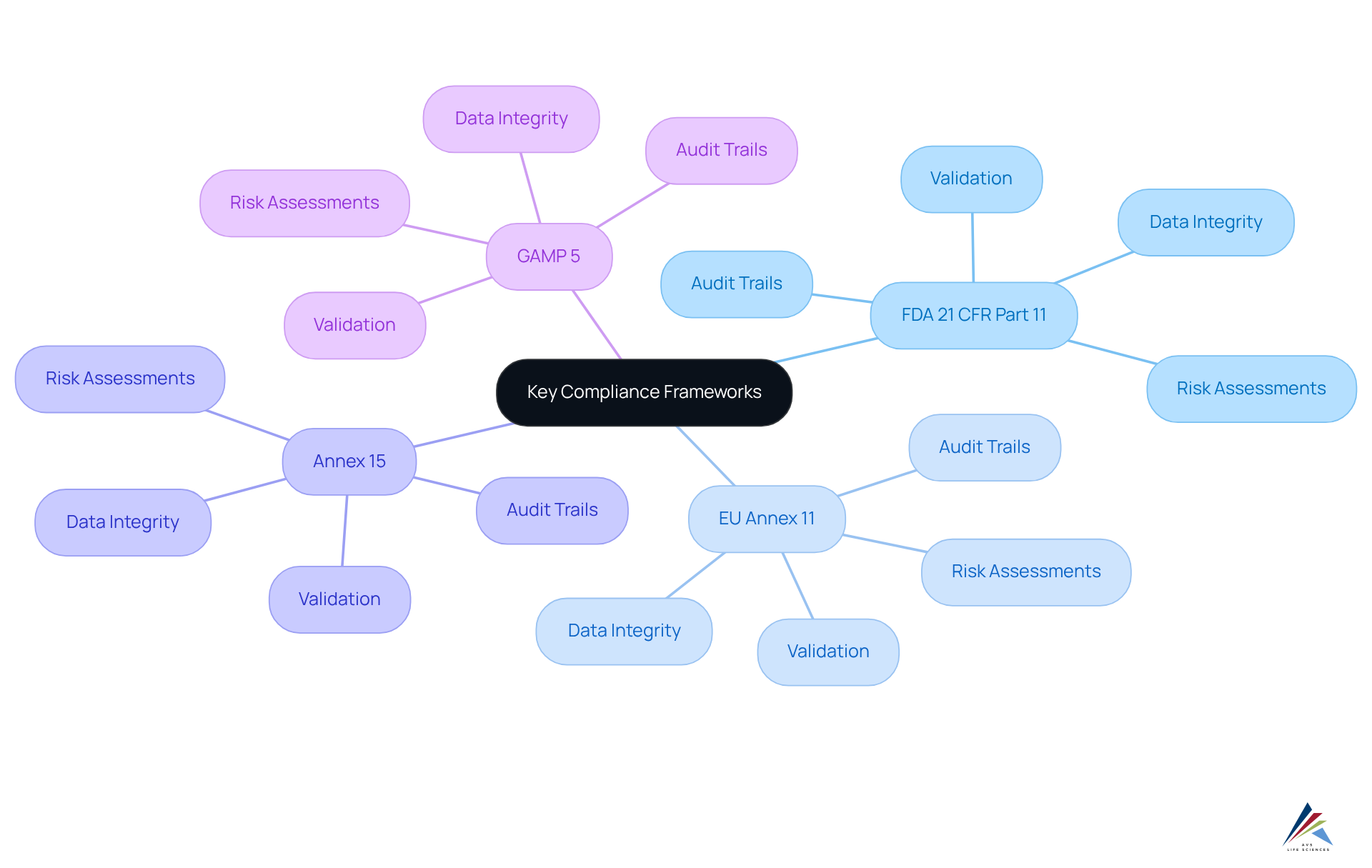
Understand Key Compliance Frameworks: FDA 21 CFR Part 11, EU Annex 11, Annex 15, and GAMP 5
Navigating compliance in the life sciences sector necessitates a comprehensive understanding of several key frameworks.
The criteria for to be considered trustworthy and reliable, as established by , , Annex 15, and , equate them to traditional paper records. Essential requirements include of the framework, comprehensive , and stringent user access controls.
EU Annex 11, along with FDA 21 CFR Part 11, emphasizes the within , as outlined in Annex 15 and GAMP 5. It adopts a lifecycle perspective on validation, underscoring the critical significance of data integrity throughout the operational phases of the framework. Notably, the proposed changes to Annex 11, set for implementation in 2025, introduce obligatory standards for audit trails and risk assessments, indicating a considerable shift in regulatory expectations.
Annex 15, along with FDA 21 CFR Part 11, EU Annex 11, and GAMP 5, outlines the criteria for the qualification and validation of computerized frameworks, ensuring adherence to quality and regulatory standards. It aligns closely with the principles outlined in the FDA 21 CFR Part 11, EU Annex 11, Annex 15, and GAMP 5, reinforcing the necessity for robust validation processes.
GAMP 5 provides direction on the validation of automated processes, aligning with the guidelines of FDA 21 CFR Part 11, EU Annex 11, and Annex 15 while promoting a risk-based approach to compliance. It emphasizes the importance of documentation and lifecycle management of computerized systems, ensuring that all critical functionalities are validated and maintained.
Recent studies indicate that adherence to EU Annex 11 significantly enhances , resulting in improved audit trails and reduced risks of citations. Successful case studies reveal that organizations applying these guidelines not only satisfy regulatory standards but also achieve operational efficiencies and cost reductions. For instance, companies utilizing a Part 11-compliant Electronic Quality Management System (EQMS) report faster resolution of deviations and enhanced readiness for inspections.
Grasping these frameworks is crucial for mastering regulations, as they provide essential guidelines to ensure data integrity and compliance in electronic environments.
Implement Step-by-Step Compliance Strategies
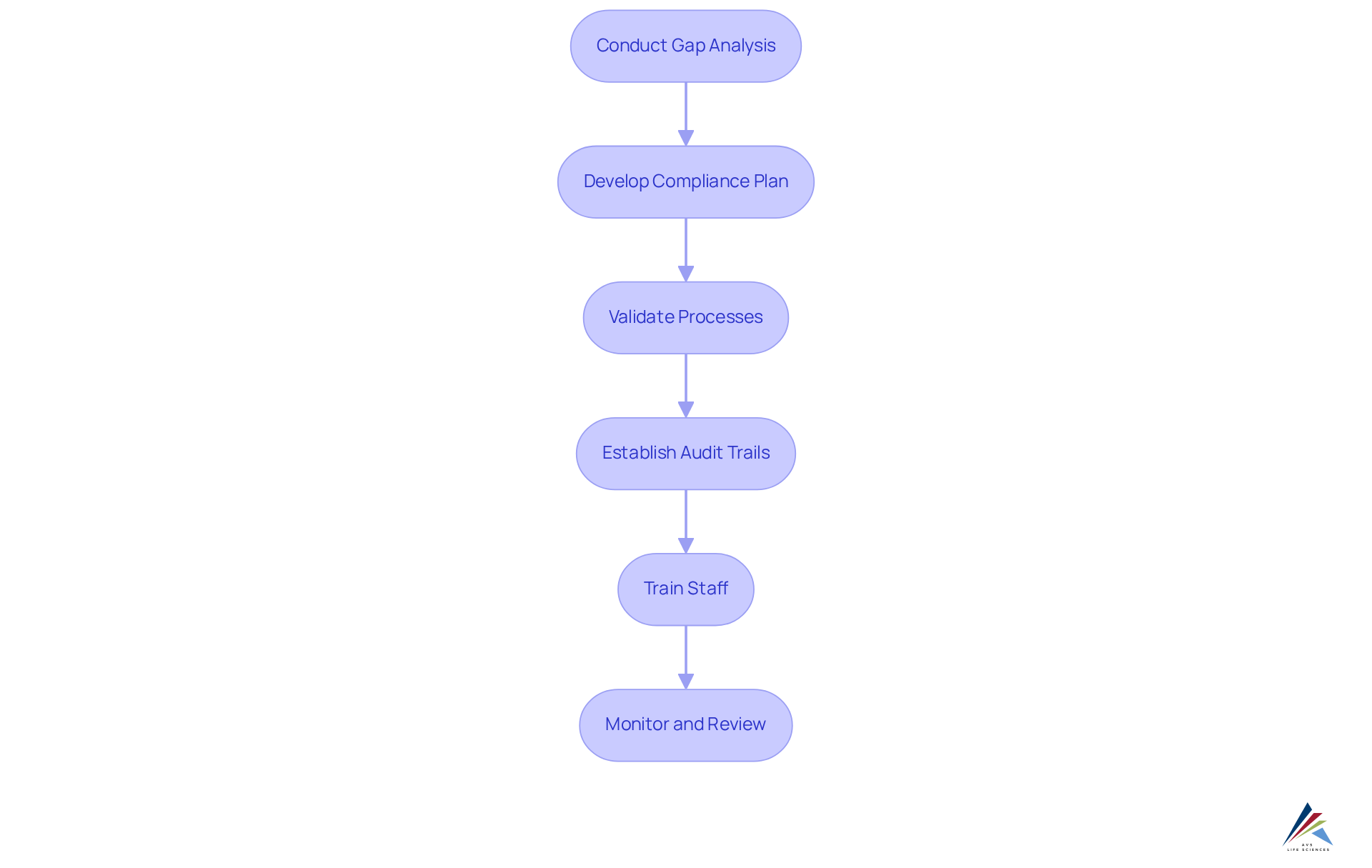
To implement effective , follow these steps:
- Conduct a by evaluating your current systems against the requirements of FDA 21 CFR Part 11, EU Annex 11, Annex 15, and GAMP 5. This analysis is crucial for identifying specific areas where your organization may not meet the regulatory standards set forth in FDA 21 CFR Part 11, EU Annex 11, Annex 15, and GAMP 5, thereby allowing for targeted improvements.
- Develop a : Next, formulate a comprehensive plan that outlines how to address the identified gaps. This plan should encompass timelines, assigned duties, and specific actions necessary to achieve adherence.
- Validate Processes: Ensure that all computerized processes are . It is essential to document the validation process carefully and maintain comprehensive records of all tests conducted to demonstrate adherence.
- Establish : Implement systems that automatically generate audit trails for all electronic records. This step is essential for demonstrating adherence during , ensuring transparency and accountability.
- Train Staff: Provide extensive training for all employees regarding regulatory requirements and the significance of . Regular training sessions are vital for promoting a culture of adherence and ensuring ongoing commitment to protocols.
- Monitor and Review: Finally, continuously observe adherence efforts and regularly assess processes to ensure sustained conformity to regulations. Be prepared to modify your adherence strategy based on results from audits and inspections, encouraging a proactive approach to management.
Overcome Compliance Challenges: Tools and Best Practices
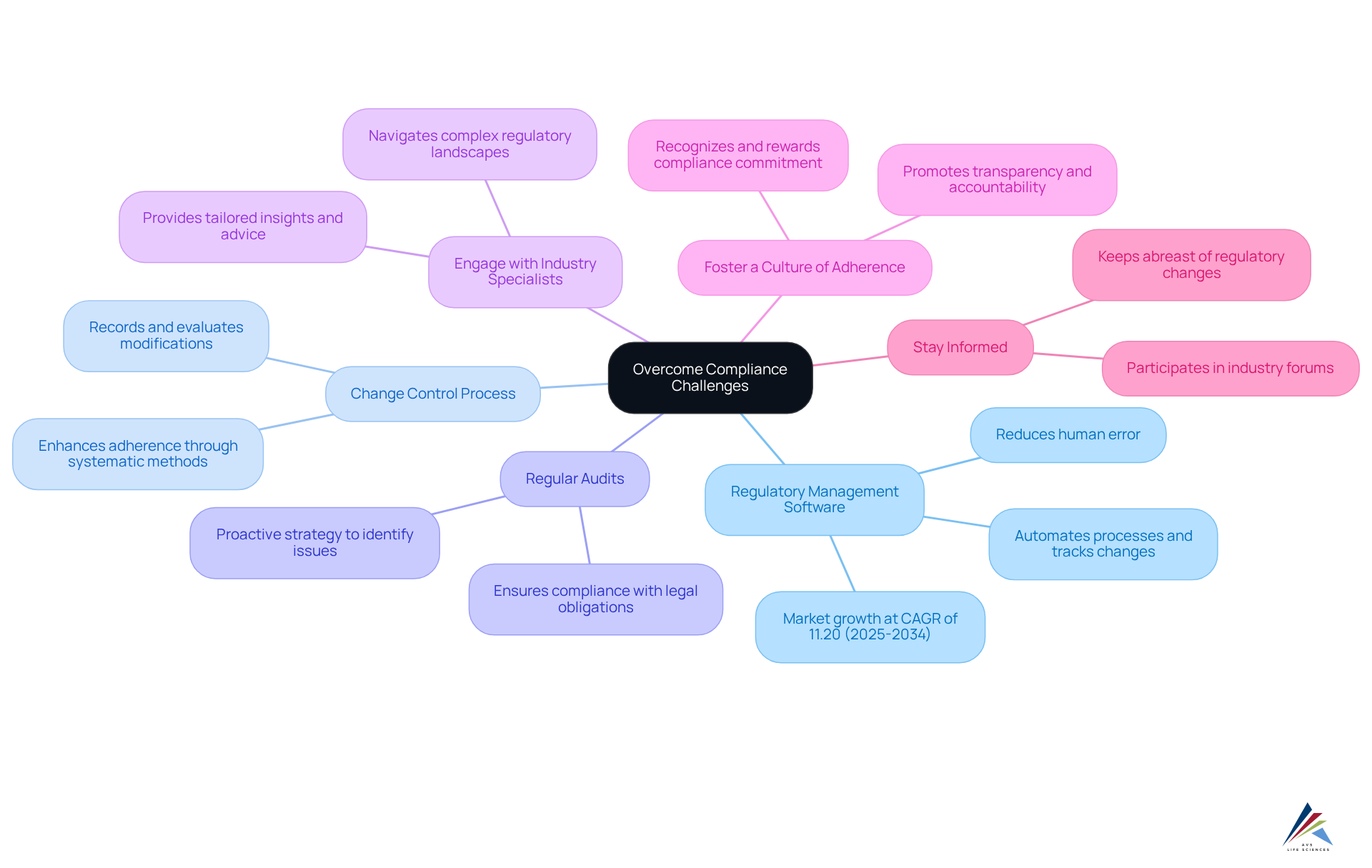
To effectively navigate compliance challenges in the pharmaceutical sector, organizations must adopt that ensure adherence to regulations and .
- Utilize : Investing in advanced is crucial. This technology automates processes, tracks changes, and maintains essential documentation, significantly reducing the risk of human error. Notably, the worldwide regulatory management software market is anticipated to expand at a CAGR of 11.20% from 2025 to 2034, underscoring the growing dependence on technology for adherence.
- Implement a : Establishing a robust change control process is vital for managing modifications to systems and processes. This guarantees that all changes are meticulously recorded and evaluated for their impact on regulations. Efficient change control procedures have been shown to enhance adherence by providing a systematic method for handling alterations, thereby reducing risks associated with non-conformity.
- Conduct : Scheduling regular internal audits is essential for evaluating adherence to regulations. This proactive strategy helps identify potential issues before they escalate into major problems, ensuring that organizations remain compliant with legal obligations.
- Engage with Industry Specialists: or industry experts can provide tailored insights and advice relevant to your organization’s needs. Their expertise is invaluable in navigating complex regulatory landscapes and effectively implementing best practices.
- Foster a : Cultivating a culture of adherence within the organization is crucial. By promoting transparency and accountability, as well as recognizing and rewarding staff who demonstrate commitment to regulatory practices, organizations can significantly enhance their overall adherence efforts.
- Stay Informed: and industry standards is essential. Subscribing to relevant publications and participating in industry forums can help organizations stay informed about best practices and regulatory trends, ensuring they adapt to evolving requirements efficiently.
By implementing these strategies, pharmaceutical companies can , ultimately leading to improved operational efficiency and a reduced risk of regulatory violations.
Conclusion
Mastering compliance with FDA 21 CFR Part 11, EU Annex 11, and GAMP 5 is not merely beneficial but essential for organizations operating within the life sciences sector. These frameworks establish rigorous standards for electronic records and signatures, underscoring the critical importance of data integrity and validation throughout the lifecycle of computerized processes. Understanding and implementing these regulations is crucial for maintaining compliance and safeguarding the integrity of data in our increasingly digital world.
Key arguments presented highlight the necessity of:
- Thorough gap analyses
- Comprehensive compliance plans
- Effective training programs to ensure adherence to these regulatory standards
Furthermore, the importance of:
- Utilizing advanced regulatory management software
- Establishing robust change control processes
- Fostering a culture of adherence within organizations
cannot be overstated. By integrating these practices, companies can enhance their operational efficiency while minimizing the risks associated with non-compliance.
Ultimately, the significance of these compliance frameworks is profound. As regulatory expectations evolve, particularly with the anticipated changes to Annex 11, organizations must remain vigilant and proactive in their compliance efforts. Embracing these guidelines not only ensures regulatory adherence but also fosters a culture of quality and integrity that can lead to long-term success in the life sciences industry.
Frequently Asked Questions
What are the key compliance frameworks in the life sciences sector?
The key compliance frameworks include FDA 21 CFR Part 11, EU Annex 11, Annex 15, and GAMP 5.
What are the main requirements for electronic records and signatures under these frameworks?
Electronic records and signatures must be trustworthy and reliable, which requires rigorous validation, comprehensive audit trails, and stringent user access controls.
How do EU Annex 11 and FDA 21 CFR Part 11 relate to Good Manufacturing Practices (GMP)?
Both EU Annex 11 and FDA 21 CFR Part 11 emphasize the validation of computerized processes within GMP, as highlighted in Annex 15 and GAMP 5, focusing on data integrity throughout the operational phases.
What changes are proposed for EU Annex 11 set to be implemented in 2025?
The proposed changes introduce obligatory standards for audit trails and risk assessments, indicating a significant shift in regulatory expectations.
What does Annex 15 outline regarding computerized frameworks?
Annex 15 outlines the criteria for the qualification and validation of computerized frameworks, ensuring adherence to quality and regulatory standards.
What is the focus of GAMP 5 in terms of compliance?
GAMP 5 provides guidance on the validation of automated processes, promoting a risk-based approach to compliance and emphasizing the importance of documentation and lifecycle management.
How does adherence to EU Annex 11 impact data integrity practices?
Adherence to EU Annex 11 significantly enhances data integrity practices, resulting in improved audit trails and reduced risks of citations.
What benefits have organizations experienced by following these compliance guidelines?
Organizations that apply these guidelines report operational efficiencies, cost reductions, faster resolution of deviations, and enhanced readiness for inspections.
