Master FAT/SAT for Equipment Qualification in Pharma Compliance

Overview
The article underscores the critical importance of Factory Acceptance Testing (FAT) and Site Acceptance Testing (SAT) within the equipment qualification process for pharmaceutical compliance. These testing phases are not merely procedural; they are essential for ensuring that equipment adheres to regulatory standards and functions correctly. By implementing FAT and SAT, organizations can significantly mitigate risks associated with equipment failure, thereby fostering operational excellence in the pharmaceutical sector. This proactive approach not only safeguards compliance but also enhances overall performance, making it imperative for industry stakeholders to prioritize these testing phases.
Introduction
In the intricate world of pharmaceutical manufacturing, ensuring equipment reliability and compliance is paramount. Factory Acceptance Testing (FAT) and Site Acceptance Testing (SAT) serve as critical checkpoints that validate machinery before and after installation, safeguarding against costly operational setbacks.
However, many organizations grapple with effectively navigating these processes, often leaving them vulnerable to compliance failures and inefficiencies.
How can companies master FAT and SAT to not only meet regulatory standards but also enhance their operational excellence?
By understanding the complexities of these testing phases, organizations can implement robust strategies that not only ensure compliance but also drive operational success.
Understand FAT and SAT: Definitions and Importance
Tech transfer CQV, including FAT/SAT, are indispensable components in the equipment qualification process of devices within the pharmaceutical sector.
FAT is executed at the manufacturer's facility prior to the apparatus being dispatched to the operational site. This testing phase is critical as it and operates correctly under controlled conditions. Early identification of potential issues during FAT significantly mitigates the risk of costly delays in subsequent installation and operational stages.
Conversely, SAT takes place after the apparatus has been installed at the location. This phase is essential for ensuring that the apparatus functions as intended within its actual working environment, thereby confirming that it adheres to the operational requirements established during FAT.
Tech transfer CQV, along with FAT and SAT, is vital for compliance with regulatory standards, as they validate that machinery is capable of consistently producing high-quality products. A comprehensive understanding of these processes is crucial for successful equipment qualification, including tech transfer CQV and FAT/SAT, ultimately supporting adherence to regulations and fostering operational excellence in pharmaceutical manufacturing.

Plan Your FAT/SAT: Key Steps and Considerations
Effective planning for tech transfer cqv, FAT/SAT, and equipment qualification is essential for ensuring adherence and operational efficiency in the pharmaceutical industry. Consider the following key steps:
- Define Objectives: Clearly articulate the goals for both FAT and SAT. This encompasses identifying essential parameters that need to be tested and validated to guarantee adherence to regulatory standards, particularly concentrating on User Requirements Specifications (URS) that effectively direct the testing process.
- Develop a Test Plan: Create a comprehensive test plan that outlines the scope of testing, acceptance criteria, and a detailed schedule. Involving all stakeholders in this process is essential to align expectations and responsibilities. A well-defined test plan is integral to the overall computer system validation process, ensuring that all necessary steps are followed, including adherence to the V-Model from the GAMP 5 Guide.
- Choose the Appropriate Team: Gather a proficient group with knowledge in machinery operation, quality assurance, and regulatory adherence. This team will be responsible for executing the tests and meticulously documenting the results, ensuring that all actions align with the standards set forth in the guidelines.
- Prepare Documentation: Ensure that all necessary documentation, including protocols, test scripts, and regulatory checklists, is prepared in advance. Comprehensive documentation is crucial throughout FAT and SAT, serving as a critical reference during testing and is vital for regulatory submissions. This documentation should reflect the results of Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) testing, and should be organized in a checklist format to reinforce a structured approach.
- Conduct Risk Assessment: Identify potential risks related to the tools and the testing process. Develop proactive mitigation strategies to address these risks, ensuring a smoother testing experience. This step is essential to guarantee that the system will continue functioning as intended after being subjected to adverse circumstances.
By adhering to these steps, organizations can establish a robust framework that lays the groundwork for effective tech transfer cqv, FAT/SAT, and equipment qualification execution, ultimately leading to significant time and cost savings while ensuring compliance with Good Manufacturing Practices (GMP). Effective planning can reduce the likelihood of issues arising during on-site installation, further enhancing operational efficiency. For instance, conducting FAT can save significant time and costs compared to addressing issues during on-site installation, reinforcing the value of thorough planning.

Execute FAT/SAT: Step-by-Step Process
Conducting Factory Acceptance Testing (FAT) and Site Acceptance Testing (SAT) is crucial for the tech transfer CQV and equipment qualification of apparatus in the pharmaceutical sector. To effectively navigate these processes, organizations can follow a structured approach:
-
Conduct FAT:
- Setup: Confirm that the equipment is configured according to the manufacturer's specifications, ensuring all components are in place for testing.
- Perform Tests: Execute the pre-defined test scripts developed during the planning phase. It is crucial to document all results meticulously, noting any deviations from expected outcomes. A comprehensive FAT/SAT checklist for tech transfer CQV should include performance criteria and compliance with industry standards, focusing on equipment qualification.
- Review Results: Analyze the results to determine if the apparatus meets the acceptance criteria. If issues arise, collaborate with the manufacturer to address and resolve them before shipment, thereby minimizing risks and ensuring customer satisfaction. A well-executed FAT can uncover design flaws and streamline integration, ultimately saving time and costs.
-
Prepare for SAT:
- Installation: Upon arrival at the site, ensure that the equipment is installed according to the manufacturer's guidelines, adhering to all safety and operational protocols.
- Conduct SAT:
- Operational Testing: Perform the tests outlined in the SAT plan, focusing on how the equipment operates within its intended environment. This phase is critical for tech transfer CQV, FAT/SAT, and equipment qualification to validate installation and functionality under real-world conditions.
- Documentation: Record all findings, including discrepancies and corrective actions taken. Thorough documentation during SAT, as part of tech transfer CQV and equipment qualification, is essential for adherence to (GMP) and serves as evidence of functionality.
- Final Review: After completing SAT, conduct a comprehensive review of all documentation to ensure compliance with regulatory requirements. This encompasses confirming that all essential instrumentation has been calibrated and that the devices are prepared for operational use.
By following these steps, organizations can effectively implement tech transfer CQV, FAT/SAT, which results in successful equipment qualification and improved operational reliability. Recent case studies highlight that companies implementing rigorous FAT and SAT processes have achieved high success rates, significantly reducing the likelihood of unexpected issues during implementation.
Troubleshoot Common Issues in FAT/SAT Execution
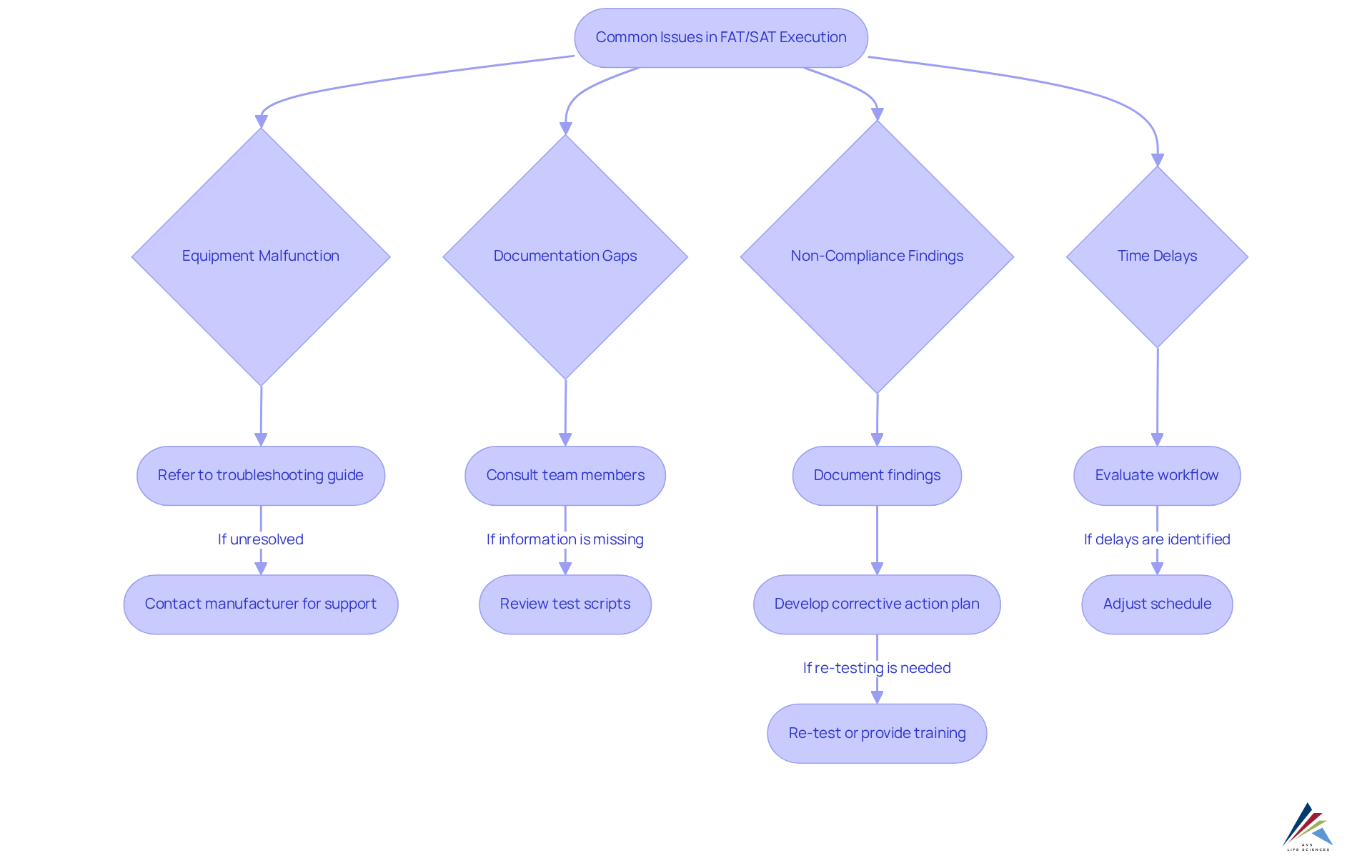
During the execution of tech transfer cqv, FAT/SAT, and equipment qualification, various challenges can arise. Identifying these prevalent issues and implementing effective solutions is essential for ensuring compliance and operational success.
- Equipment Malfunction: When equipment fails to operate as expected during FAT or SAT, it is crucial to refer to the manufacturer's troubleshooting guide. Should problems persist, promptly contact the manufacturer for technical support to ensure a timely resolution.
- Documentation Gaps: Thorough documentation of all test results is vital. If any information is missing, address it immediately by consulting team members or reviewing test scripts to guarantee adherence and traceability.
- Non-Compliance Findings: In the event of any testing aspect failing to meet regulatory requirements, meticulously document the findings and develop a corrective action plan. This may necessitate re-testing or additional training for the team to ensure compliance with standards.
- Time Delays: If testing extends beyond the anticipated timeframe, evaluate the workflow to identify bottlenecks. Adjust the schedule as necessary while ensuring that quality and regulatory standards are not compromised.
By proactively addressing these common issues and implementing effective strategies, you can significantly enhance the efficiency and success of your equipment qualification during the tech transfer CQV, FAT, and SAT processes. Ultimately, this leads to and fosters a culture of excellence within your organization.
Conclusion
Mastering Factory Acceptance Testing (FAT) and Site Acceptance Testing (SAT) is essential for ensuring equipment qualification and compliance within the pharmaceutical industry. These processes validate that equipment meets specified requirements and confirm its operational efficacy in real-world settings. By understanding and effectively implementing FAT and SAT, organizations can significantly mitigate risks, streamline operations, and enhance product quality.
The article outlines the critical steps involved in planning and executing FAT and SAT, emphasizing the importance of:
- Defining objectives
- Developing comprehensive test plans
- Preparing thorough documentation
Key insights include the necessity of assembling a knowledgeable team and conducting risk assessments to preemptively address potential challenges. Moreover, troubleshooting common issues during execution ensures that compliance standards are met, ultimately fostering operational excellence.
In a highly regulated industry where adherence to standards is paramount, prioritizing the mastery of FAT and SAT processes can lead to substantial benefits. Organizations are encouraged to invest time and resources in refining these practices, as doing so not only promotes compliance but also contributes to the overall reliability and success of pharmaceutical operations. Embracing these methodologies is not just a regulatory necessity; it is a strategic imperative for achieving excellence in manufacturing and product delivery.
Frequently Asked Questions
What are FAT and SAT in the context of equipment qualification?
FAT (Factory Acceptance Testing) and SAT (Site Acceptance Testing) are critical components of the equipment qualification process in the pharmaceutical sector. FAT is conducted at the manufacturer's facility before the equipment is shipped, while SAT occurs after the equipment has been installed at the operational site.
What is the purpose of FAT?
The purpose of FAT is to confirm that the equipment meets specified requirements and operates correctly under controlled conditions before it is dispatched. It allows for early identification of potential issues, which helps to mitigate the risk of costly delays during installation and operational stages.
What does SAT aim to achieve?
SAT aims to ensure that the equipment functions as intended within its actual working environment after installation. It confirms that the equipment adheres to the operational requirements established during FAT.
Why are FAT and SAT important for compliance?
FAT and SAT are important for compliance with regulatory standards because they validate that machinery is capable of consistently producing high-quality products. This compliance is essential for maintaining operational excellence in pharmaceutical manufacturing.
How do FAT and SAT contribute to tech transfer CQV?
FAT and SAT are integral to tech transfer CQV (Commissioning, Qualification, and Validation) as they provide a comprehensive understanding of the equipment's performance and capabilities. This understanding supports successful equipment qualification and adherence to regulations.
