Master EU GMP Compliance: 4 Essential Steps for Success

Overview
The article delineates four essential steps for mastering EU GMP compliance:
- Understanding GMP fundamentals
- Identifying key regulations
- Implementing effective compliance strategies
- Establishing continuous monitoring processes
These steps are bolstered by comprehensive discussions on quality management, staff training, and regular audits. A robust compliance framework not only meets regulatory requirements but also significantly enhances product safety and operational efficiency within pharmaceutical manufacturing. By addressing compliance challenges head-on and providing actionable insights, this overview serves as a critical guide for those seeking to navigate the complexities of EU GMP compliance effectively.
Introduction
Good Manufacturing Practice (GMP) serves as the backbone of pharmaceutical safety and quality, ensuring that products are consistently produced to the highest standards. In light of increasing scrutiny and evolving regulations within the pharmaceutical industry, it becomes paramount for organizations to understand the essential steps for achieving compliance with EU GMP. This understanding not only safeguards their products but also protects their reputations.
However, navigating the complexities of EU GMP presents significant challenges. This leads many to ponder: what are the most effective strategies for ensuring compliance and fostering a culture of continuous improvement in this critical field? By exploring these strategies, organizations can enhance their compliance efforts and drive sustainable success.
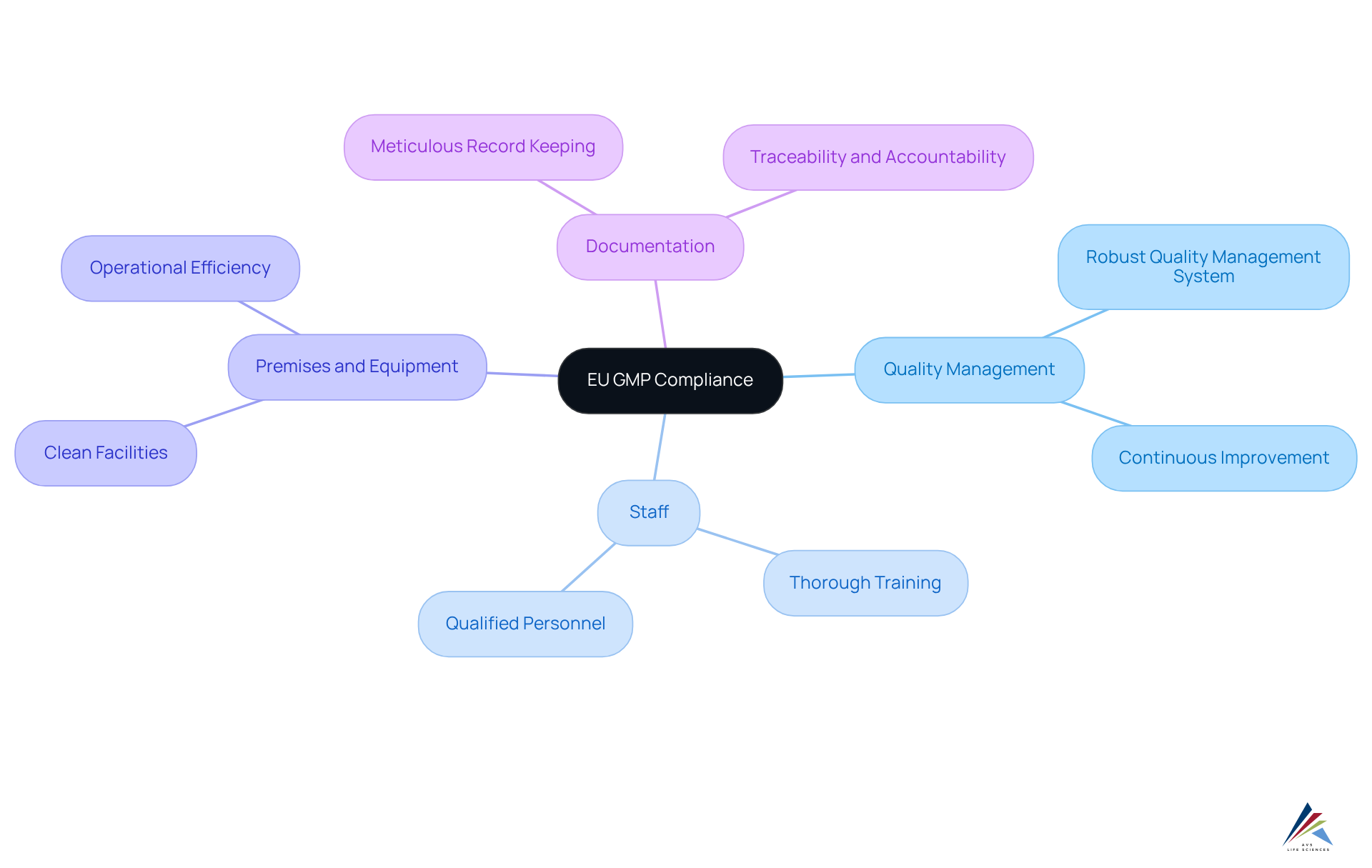
Understand the Fundamentals of EU GMP Compliance
encompasses the systems and processes that guarantee products are consistently produced and regulated according to established excellence standards. The primary objective of is to reduce risks in that cannot be addressed solely through final product testing. Key principles include:
- Quality Management: Implementing a robust that integrates all facets of production, ensuring and adherence to standards.
- Staff: Ensuring that all staff are thoroughly trained and qualified, as is crucial for minimizing errors and maintaining adherence.
- Premises and Equipment: Maintaining clean, suitable facilities and equipment to prevent contamination and ensure operational efficiency.
- Documentation: Keeping of all processes and changes, which is essential for traceability and accountability.
Grasping these fundamentals is essential for and ensuring the in the market. As emphasized by industry leaders, a not only enhances product safety but also fosters a culture of continuous improvement, ultimately benefiting both producers and consumers.
Identify Key EU GMP Requirements and Regulations
To achieve compliance with EU GMP, organizations must thoroughly understand several pivotal regulations:
- : This comprehensive document delineates the principles and guidelines governing GMP for medicinal products within the EU. It includes essential criteria for manufacturing, quality control, and distribution, ensuring that all operations align with regulatory expectations. to assist entities in understanding and applying these guidelines effectively.
- Directive 2003/94/EC: This directive outlines specific requirements for , reinforcing the standards established in EudraLex Volume 4 and ensuring that entities maintain high-quality production methods. to meet these requirements.
- Annex 1: Specifically targeting the manufacture of sterile medicinal products, Annex 1 outlines stringent requirements for facilities, equipment, and methods to comply with EU GMP. Compliance with EU GMP standards is crucial for maintaining the integrity and safety of sterile products. AVS Life Sciences has a proven track record of assisting organizations in meeting these stringent criteria, reinforcing their commitment to quality.
- Annex 11: This annex focuses on , highlighting the necessity for robust validation and data integrity in digital processes according to EU GMP standards. As the sector progressively depends on technology, adherence to Annex 11 is vital for ensuring that electronic records and systems comply with EU GMP standards and facilitate operational efficiency. to guarantee adherence to these regulations.
- EudraGMDP Database: This publicly accessible EU database offers guidance on good manufacturing practice and good distribution practice, serving as an . AVS Life Sciences can assist entities on how to effectively utilize this resource.
- : These agreements promote the exchange of information on inspections and product defects, improving international cooperation and regulatory effectiveness in GMP adherence. AVS Life Sciences can assist entities in navigating these agreements to enhance their adherence strategies.
Familiarity with is vital for businesses to ensure that every aspect of production meets the required standards, ultimately safeguarding product quality and patient safety. By collaborating with AVS Life Sciences, entities can improve their regulatory efforts and guarantee adherence to these essential regulations.

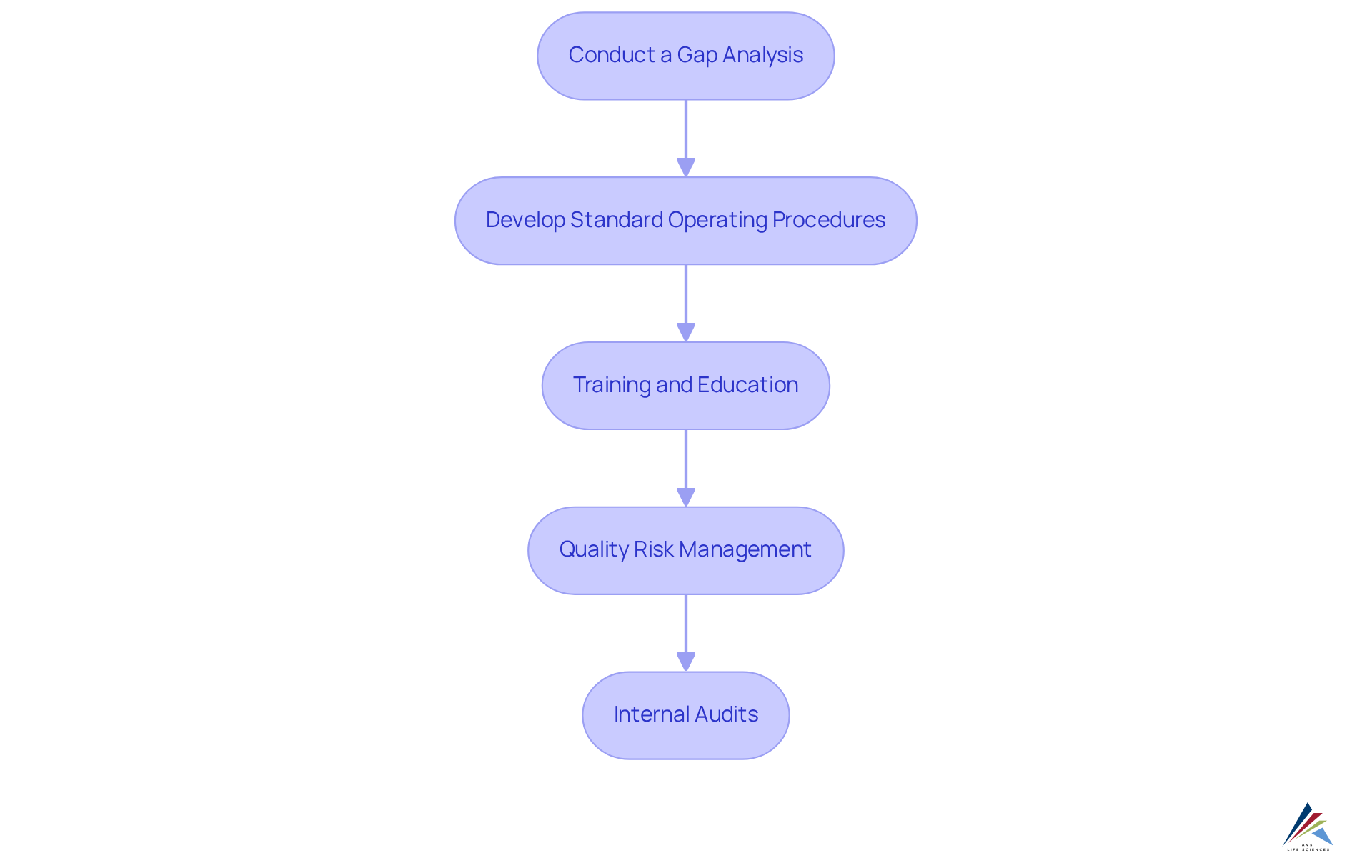
Implement Effective Compliance Strategies and Best Practices
To implement effective compliance strategies, organizations must adopt best practices that address compliance challenges head-on:
- : Systematically assess current practices against EU GMP requirements to identify areas needing improvement. Routine gap evaluations are crucial, even after initial adherence is achieved, as they reveal new gaps resulting from changes in procedures or regulations.
- Develop : Create detailed SOPs for all processes, ensuring they are tailored to the complexity of tasks and regularly reviewed and updated. Effective SOPs are essential for documenting routine activities and ensuring consistency in care standards, ultimately enhancing .
- : Provide for all employees to ensure they understand EU GMP principles and their specific roles in maintaining adherence. Continuous training is not only a regulatory requirement but also vital for fostering a culture of quality within the organization. Training gaps frequently result in adherence problems, making it essential to invest in .
- : Implement a robust risk management framework to identify, assess, and mitigate risks associated with . This proactive strategy enables entities to prioritize enhancements and distribute resources efficiently, improving overall adherence and product excellence.
- : Regularly carry out internal audits to assess adherence and identify areas for enhancement. These audits should include a review of training records and SOP adherence, as they are critical for maintaining high-quality standards and ensuring readiness for external inspections.
By implementing these strategies, entities can create a strong that not only fulfills legal obligations but also improves overall management standards. The significance of SOPs in guaranteeing adherence is underscored by industry leaders, who emphasize that well-documented procedures are crucial for upholding operational integrity and patient safety.
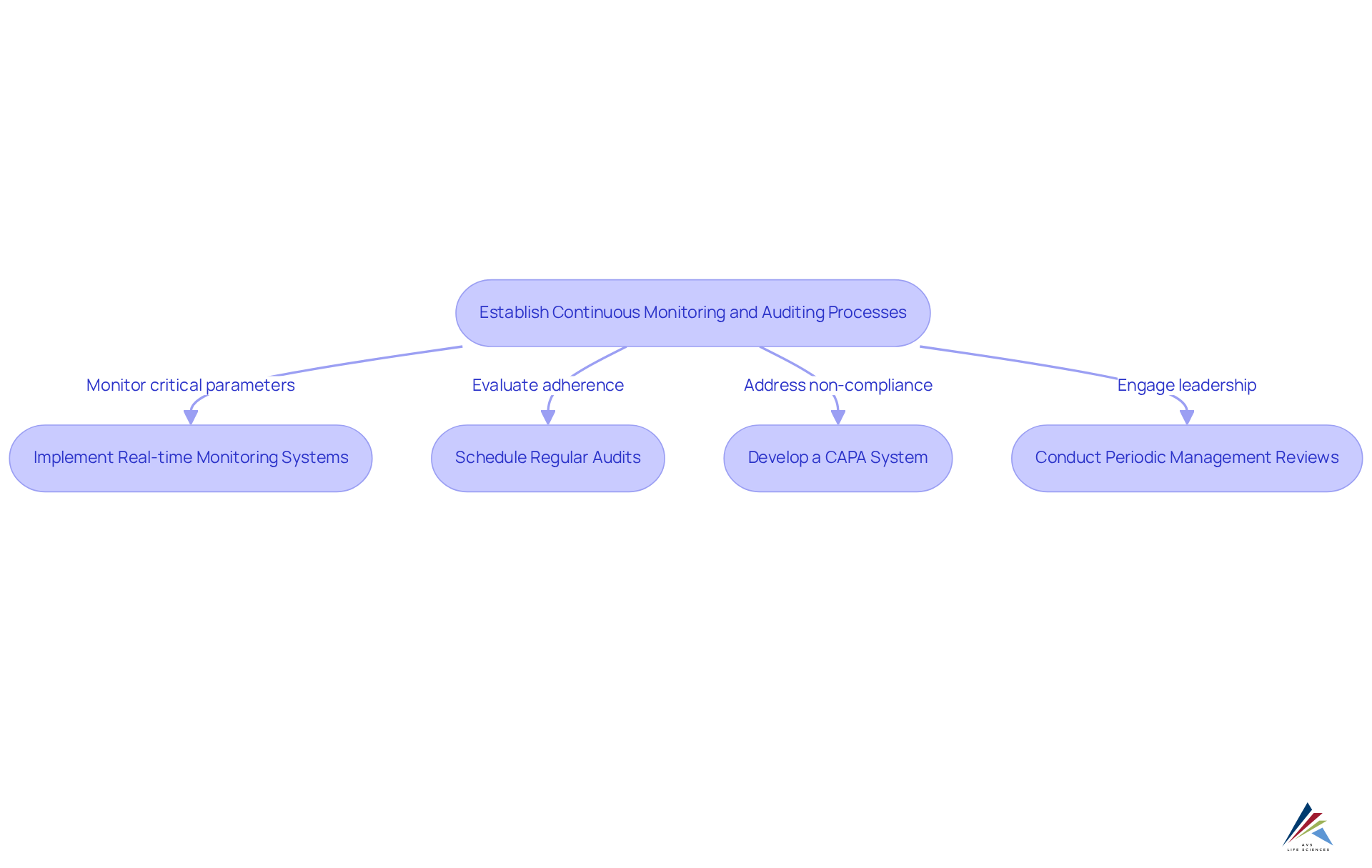
Establish Continuous Monitoring and Auditing Processes
To ensure ongoing , organizations must establish robust that are integral to their . Continuous monitoring is essential; implementing for critical manufacturing parameters—such as temperature, humidity, and contamination levels—enables the proactive identification of deviations before they impact product quality.
Regular audits, both internal and external, should be scheduled at defined intervals to evaluate adherence to . Data indicates that organizations performing regular audits experience a significant reduction in , with many achieving zero findings during inspections.
Furthermore, developing a comprehensive system is crucial. This system should promptly address any non-compliance issues identified during audits, ensuring that corrective actions are not only implemented but also monitored for effectiveness, thereby fostering a culture of continuous improvement.
Periodic and audit findings are also necessary. This practice guarantees that leadership remains knowledgeable and engaged with EU GMP requirements, facilitating prompt modifications to methods and strategies.
By establishing these processes, organizations can cultivate a , leading to enhanced quality management and improved outcomes in pharmaceutical manufacturing.
Conclusion
Mastering EU GMP compliance is essential for organizations within the pharmaceutical industry, as it ensures that products are manufactured to the highest safety and quality standards. By comprehensively understanding the core principles of Good Manufacturing Practice, organizations can significantly mitigate the risks associated with production processes and enhance overall product integrity.
This article outlines the critical steps necessary for achieving compliance, including:
- Grasping fundamental regulations
- Implementing effective strategies
- Establishing continuous monitoring and auditing processes
Key insights emphasize the importance of:
- Quality management systems
- Thorough staff training
- Rigorous documentation practices
Furthermore, the necessity of conducting regular audits and maintaining a proactive approach to risk management is highlighted as crucial for sustaining compliance and fostering a culture of quality.
Given the complexities surrounding EU GMP compliance, organizations are strongly encouraged to prioritize these practices—not only to meet regulatory obligations but also to ensure patient safety and product excellence. By embracing a commitment to continuous improvement and leveraging available resources, companies can navigate the challenges of compliance effectively and contribute to a safer pharmaceutical landscape.
Frequently Asked Questions
What is the purpose of EU GMP compliance?
The purpose of EU GMP compliance is to reduce risks in pharmaceutical production that cannot be addressed solely through final product testing, ensuring products are consistently produced and regulated according to established excellence standards.
What are the key principles of Good Manufacturing Practice (GMP)?
The key principles of GMP include Quality Management, Staff training and qualification, maintaining clean Premises and Equipment, and thorough Documentation of processes and changes.
Why is a robust quality management system important in GMP?
A robust quality management system is important because it integrates all facets of production, ensuring continuous improvement and adherence to established standards.
How does staff training contribute to GMP compliance?
Staff training contributes to GMP compliance by minimizing errors and maintaining adherence to standards, ensuring that all personnel are thoroughly trained and qualified.
What role do premises and equipment play in GMP compliance?
Clean and suitable premises and equipment play a crucial role in preventing contamination and ensuring operational efficiency, which is essential for GMP compliance.
Why is documentation important in the context of GMP?
Documentation is important because it involves keeping meticulous records of all processes and changes, which is essential for traceability and accountability in pharmaceutical production.
How does a commitment to management impact product safety and improvement?
A commitment to management enhances product safety and fosters a culture of continuous improvement, ultimately benefiting both producers and consumers in the pharmaceutical industry.
