Master Equipment Validation: Best Practices for Compliance Officers

Overview
The article addresses the critical challenges faced by compliance officers in mastering equipment validation, underscoring its essential role in ensuring that equipment consistently meets regulatory standards and performs as intended. A systematic approach is emphasized, incorporating thorough documentation and a phased methodology:
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
This approach collectively enhances operational efficiency and compliance. It not only safeguards product quality but also ensures patient safety in the life sciences sector. By adopting these best practices, compliance officers can effectively navigate the complexities of regulatory requirements, ultimately fostering a culture of excellence within their organizations.
Introduction
In the highly regulated landscape of the pharmaceutical and biotechnology industries, the stakes are undeniably high regarding equipment validation. This systematic process not only ensures that machinery operates reliably but also meets stringent safety standards, directly influencing product quality and patient safety.
As organizations strive to uphold compliance and enhance operational efficiency, they face the challenge of navigating complex regulatory requirements while implementing best practices. These practices must not only satisfy legal obligations but also foster a culture of quality.
How can compliance officers effectively master equipment validation to mitigate risks and ensure the integrity of their products?
Define Equipment Validation and Its Importance
Equipment validation is a systematic process that verifies whether a unit consistently performs according to its intended use and meets predefined specifications. This process is vital in the pharmaceutical and biotechnology sectors, where the integrity of products can directly impact patient safety. Without equipment validation of the tools, there is a risk of creating faulty products that may not comply with safety standards. By guaranteeing that machinery functions dependably, organizations can uphold adherence to Good Manufacturing Practices (GMP) and evade expensive enforcement penalties. A well-documented equipment validation process can prevent failures that might lead to product recalls, thereby safeguarding both the company’s reputation and its financial standing.
AVS Life Sciences exemplifies a reliable partner in this field, offering comprehensive quality management and governance solutions customized for the life sciences sector, including full-service biopharmaceutical drug development. Their proficiency in enhancing GMP facilities, as illustrated in a recent case study, highlights their dedication to quality assurance and compliance with standards. In one instance, AVS Life Sciences assisted a leading biotechnology company in upgrading their manufacturing space from a Biosafety Level 1 GMP facility to a Level 2 GMP facility for lentivirus production. This successful transition not only adhered to regulatory standards but also enhanced the client's operational capabilities, allowing them to focus on developing critical medicines for patients.
Furthermore, equipment validation supports the overall quality management system by providing documented proof that instruments are operating properly, which is crucial during audits and inspections. This proactive approach enhances operational efficiency and fosters a culture of quality within the organization, ultimately leading to improved product outcomes and customer satisfaction. Statistics suggest that efficient equipment validation can lead to cycle time reductions of up to 50%, further emphasizing its significance in enhancing production processes.
Case studies emphasize the importance of equipment validation in biotechnology. For instance, a major US pharmaceutical manufacturer successfully validated an Automated Process Control System (APCS) for a new high-volume packaging machine, which included five Human-Machine Interfaces (HMIs) and three Programmable Logic Controllers (PLCs). This confirmation ensured adherence to FDA regulations and facilitated prompt New Drug Application (NDA) submissions, demonstrating the company's dedication to quality and patient safety. As Darragh Boyle mentions, "Equipment verification is not merely a compliance necessity; it’s an essential aspect of quality assurance." Such examples demonstrate that comprehensive verification procedures, particularly equipment validation, are not just compliance necessities; they are vital for guaranteeing the safety and efficacy of pharmaceutical products.
Understand Regulatory Requirements and Compliance Standards
In the life sciences sector, compliance with legal requirements is essential. Key regulations, such as the FDA's 21 CFR Part 820, which delineates Quality System Regulations, and ISO 13485, outlining the requirements for a quality management system, form the backbone of equipment validation. Compliance officers must ensure that all verification activities are meticulously aligned with these standards to prevent oversight.
AVS Life Sciences distinguishes itself as a committed ally in this field, providing customized regulatory, compliance, and quality solutions, including engineering support and guidance for regulatory submissions, specifically for the biopharmaceuticals, medical devices, and nutraceuticals industries. The FDA mandates that all apparatus used in manufacturing must undergo equipment validation to ensure it meets outlined criteria. This confirmation process is not a singular occurrence; it requires continuous oversight and re-assessment as situations evolve. For instance, compliance rates for equipment validation have shown significant improvement, reflecting the industry's commitment to maintaining high standards.
Case studies demonstrate the significance of strong verification protocols. Organizations that have adopted comprehensive equipment validation strategies in accordance with ISO 13485, backed by AVS Life Sciences' expert consulting, have reported improved operational efficiency and fewer occurrences of non-compliance. By grasping and implementing these regulatory requirements, oversight officers can create effective verification protocols that not only satisfy regulatory expectations but also reduce risks related to non-adherence.
Implement the Phased Approach: IQ, OQ, and PQ
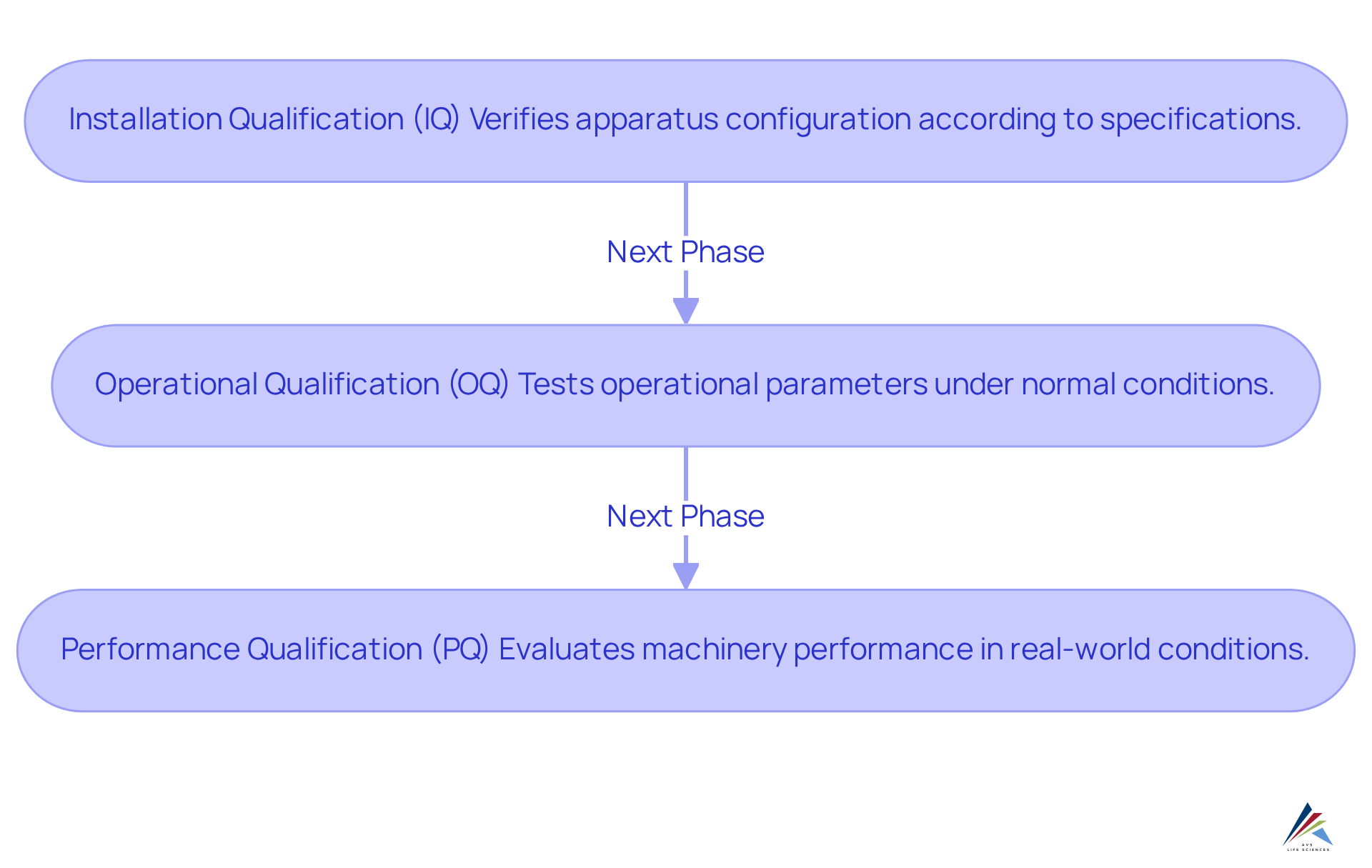
The phased approach to equipment validation consists of three essential components: Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Each phase is vital to ensuring compliance and operational efficiency:
-
Installation Qualification (IQ): This initial phase verifies that the apparatus is configured according to the manufacturer's specifications. It involves a comprehensive evaluation of the installation environment, utilities, and setup, ensuring that all components are correctly positioned and functional. Proper documentation during this phase is critical, as it provides tangible evidence that the installation meets the necessary requirements for equipment validation, aligning with regulatory expectations and the Computer System Validation (CSV) process checklist.
-
Operational Qualification (OQ): In this phase, the operational parameters of the apparatus undergo rigorous testing to confirm that it operates as intended under normal conditions. This includes assessing key factors such as temperature, pressure, and speed, ensuring that the apparatus functions within defined limits and consistently delivers reliable results. Recent trends indicate that successful OQ implementations in pharmaceutical validation have significantly enhanced operational reliability, with many organizations reporting improved compliance outcomes. Incorporating worst-case scenarios in testing has proven effective in minimizing non-compliance incidents, underscoring the importance of thorough testing methodologies as outlined in the CSV process.
-
Performance Qualification (PQ): The final phase evaluates the machinery's performance in real-world conditions, confirming that it consistently meets predefined criteria. This phase typically involves executing real production batches, allowing for a comprehensive assessment of the machinery's efficiency in delivering quality outputs. Equipment validation is crucial for demonstrating that the equipment can reliably produce products that comply with regulatory standards, thereby mitigating risks associated with non-compliance. The verification outcomes must be meticulously recorded, as this documentation serves as proof of adherence to FDA regulations and Good Automated Manufacturing Practices (GAMP).
Adopting this staged methodology not only ensures thorough verification but also emphasizes the importance of detailed documentation practices, which are essential for equipment validation in quality management systems. By maintaining a clear documentation trail, organizations can enhance their verification processes, resulting in improved compliance with regulations and operational success. Furthermore, recognizing common pitfalls in the verification process, such as inadequate testing scenarios or insufficient documentation, can further bolster compliance efforts, ensuring that organizations meet the stringent standards established by regulatory bodies.
Prioritize Documentation and Traceability in Validation
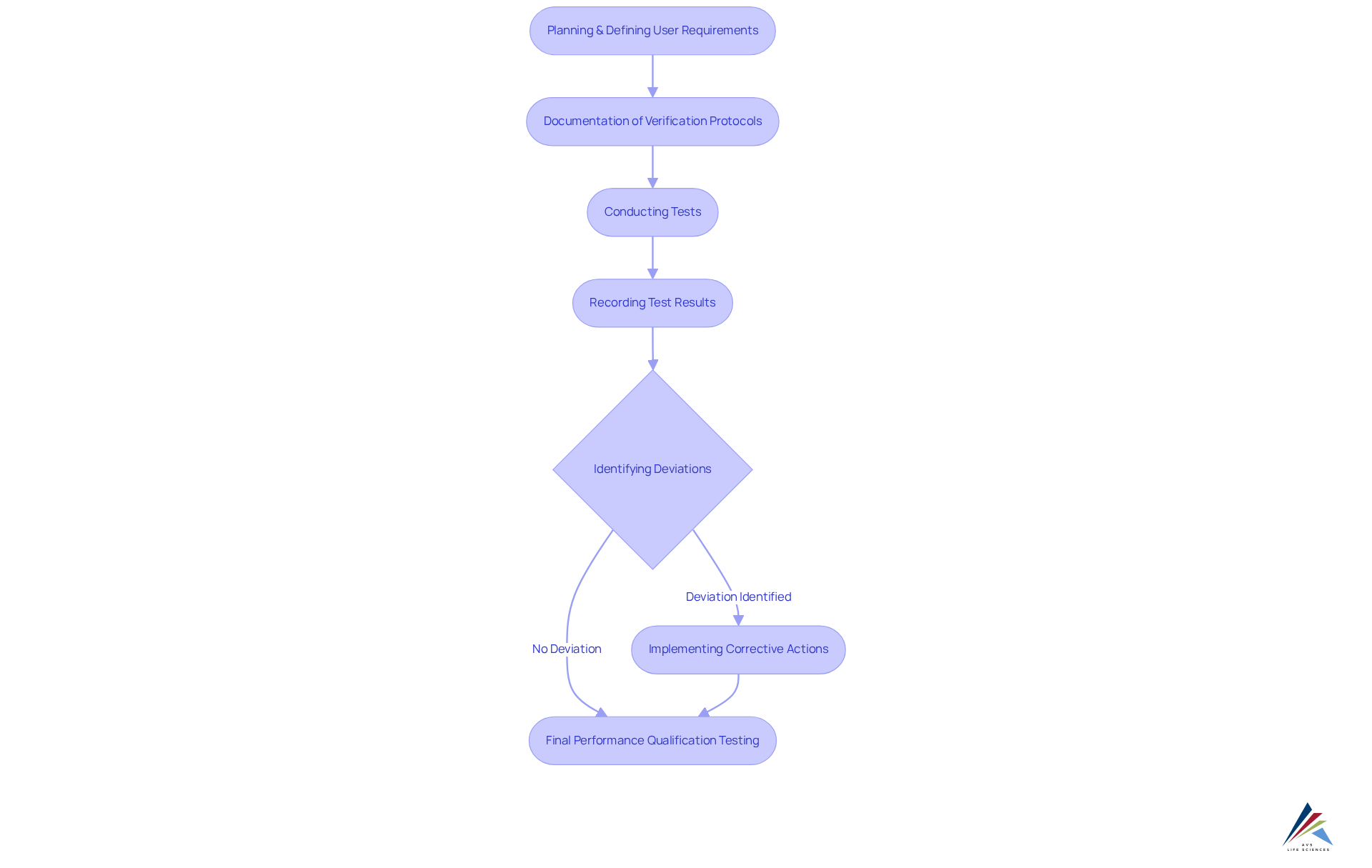
Documentation serves as the backbone of the equipment validation process, necessitating meticulous recording of every step to establish a clear audit trail. This includes verification protocols, test results, deviations, and corrective actions. Such thorough documentation not only ensures adherence to regulatory standards but also serves as a valuable reference for equipment validation and future verification efforts.
Traceability is equally vital, allowing all tools and processes to be traced back to their original specifications and verification results. This aspect becomes particularly crucial during audits, as regulators scrutinize documentation to confirm compliance. For example, if equipment fails during production, comprehensive records of its verification history can aid in identifying the root cause and demonstrate that the organization has implemented appropriate corrective actions.
The equipment validation process underscores the significance of thorough documentation at every stage, from planning and defining user requirements to installation and performance qualification testing. Recent advancements in electronic documentation systems have significantly enhanced traceability and simplified the verification process. These systems facilitate efficient record management, ensuring compliance with regulatory standards while improving overall operational efficiency. By adopting such technologies, organizations can effectively manage their validation documentation, ultimately reinforcing a robust quality management system. Prioritizing documentation and traceability not only boosts compliance but also cultivates a culture of accountability and continuous improvement within the organization.
Conclusion
Equipment validation is not merely a regulatory requirement; it is a fundamental practice that ensures the safety, efficacy, and quality of pharmaceutical products. By systematically verifying that equipment performs reliably and meets established specifications, organizations can safeguard patient health and maintain compliance with stringent industry standards. A commitment to a robust validation process ultimately enhances operational efficiency and fortifies a company’s reputation in the marketplace.
Throughout this article, key practices for effective equipment validation have been explored. The phased approach of Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) provides a structured methodology for ensuring compliance and operational integrity. Furthermore, the emphasis on thorough documentation and traceability underscores their critical roles in maintaining compliance and facilitating successful audits. Real-world case studies illustrate the tangible benefits of a rigorous validation process, showcasing improved operational outcomes and reduced risks.
In conclusion, prioritizing equipment validation is essential for compliance officers and organizations within the life sciences sector. By embracing best practices and adhering to regulatory requirements, companies can not only mitigate the risks of non-compliance but also foster a culture of quality and accountability. As the industry continues to evolve, maintaining a steadfast commitment to equipment validation will be crucial in ensuring the delivery of safe and effective products to patients.
Frequently Asked Questions
What is equipment validation?
Equipment validation is a systematic process that verifies whether a unit consistently performs according to its intended use and meets predefined specifications.
Why is equipment validation important in the pharmaceutical and biotechnology sectors?
Equipment validation is crucial in these sectors because it ensures the integrity of products, which directly impacts patient safety. It helps prevent the creation of faulty products that may not comply with safety standards.
How does equipment validation contribute to Good Manufacturing Practices (GMP)?
By ensuring that machinery functions dependably, equipment validation helps organizations uphold adherence to GMP, thereby avoiding expensive enforcement penalties.
What are the consequences of not validating equipment?
Without equipment validation, there is a risk of product failures that could lead to recalls, negatively affecting the company's reputation and financial standing.
How does AVS Life Sciences contribute to equipment validation?
AVS Life Sciences offers comprehensive quality management and governance solutions tailored for the life sciences sector, including assistance in upgrading GMP facilities and ensuring compliance with standards.
Can you provide an example of successful equipment validation?
One example is when AVS Life Sciences helped a biotechnology company upgrade from a Biosafety Level 1 GMP facility to a Level 2 GMP facility for lentivirus production, adhering to regulatory standards and enhancing operational capabilities.
How does equipment validation support quality management systems?
Equipment validation provides documented proof that instruments are operating correctly, which is essential during audits and inspections, thereby enhancing operational efficiency and fostering a culture of quality.
What impact can efficient equipment validation have on production processes?
Efficient equipment validation can lead to cycle time reductions of up to 50%, highlighting its significance in improving production processes.
What is a case study that illustrates the importance of equipment validation?
A major US pharmaceutical manufacturer validated an Automated Process Control System for a new high-volume packaging machine, ensuring adherence to FDA regulations and facilitating prompt New Drug Application submissions.
What is the overall message regarding equipment validation in the article?
Equipment validation is not just a compliance necessity; it is a vital aspect of quality assurance that guarantees the safety and efficacy of pharmaceutical products.
