Master Computer System Validation in Pharma for Compliance Success
Overview
Computer system validation (CSV) in the pharmaceutical sector is crucial for ensuring that computer technologies consistently deliver accurate results. This process safeguards data integrity and ensures compliance with regulatory requirements. Effective CSV processes, particularly those that are risk-focused and incorporate ongoing monitoring, significantly enhance operational efficiency. They maintain compliance with critical regulations such as the FDA's 21 CFR Part 11 and EU Annex 11. Ultimately, these measures are vital for ensuring product quality and patient safety. As compliance challenges continue to evolve, embracing robust CSV practices is not just advisable—it is imperative for sustaining excellence in the pharmaceutical industry.
Introduction
In an industry where precision and compliance are paramount, the significance of computer system validation (CSV) in pharmaceuticals cannot be overstated. This essential process not only safeguards data integrity but also ensures that pharmaceutical companies meet stringent regulatory requirements while prioritizing patient safety.
However, as the landscape of regulations evolves, organizations face substantial challenges in navigating the complexities of CSV. How can they enhance operational efficiency and product quality amidst these challenges?
Exploring best practices and regulatory frameworks surrounding CSV reveals critical insights into achieving compliance success in the pharmaceutical sector. By understanding and implementing effective CSV strategies, organizations can not only comply with regulations but also foster a culture of quality and safety that ultimately benefits patients and enhances their reputation in the industry.
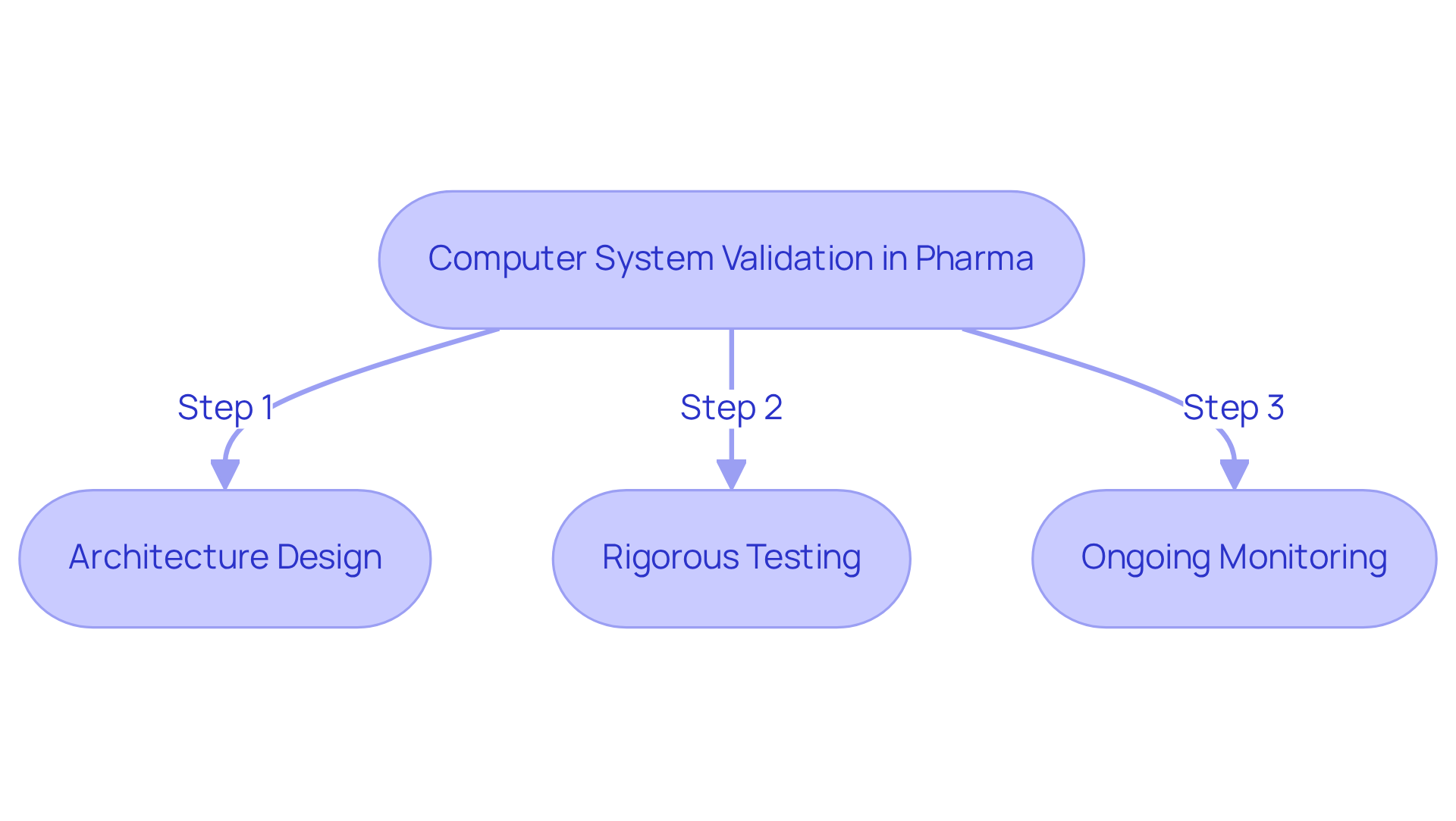
Define Computer System Validation in Pharma
Computer system validation in pharma refers to a systematic and documented process that ensures computer technologies consistently deliver results that meet defined specifications and quality standards. This verification is essential for , complying with regulatory requirements, and ensuring patient safety.
CSV involves a comprehensive series of activities, including:
- Architecture design
- Rigorous testing
- Ongoing monitoring
to confirm that systems function as intended throughout their lifecycle. By adopting effective validation practices, pharmaceutical companies can significantly improve operational efficiency, reliability, and adherence to [Good Manufacturing Practices (GMP)](https://avslifesciences.com/job-opportunities/computer-system-validation-csv) and other regulatory frameworks.
The critical role of computer system validation in pharma is highlighted by its contribution to maintaining the integrity of electronic records and ensuring that systems positively impact overall product quality and patient safety.
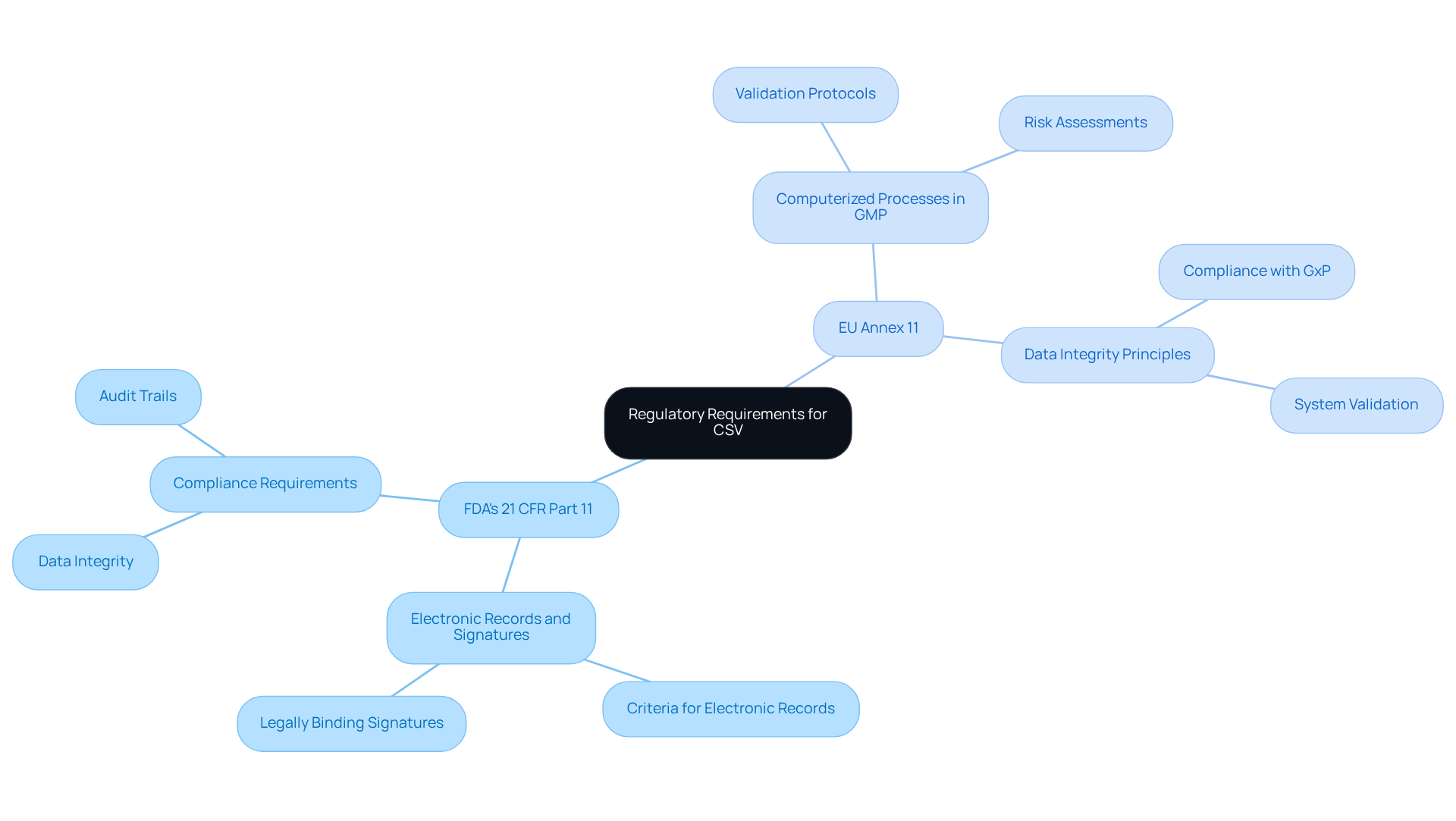
Understand Regulatory Requirements for CSV
Regulatory requirements for Computer System Validation are primarily dictated by agencies such as the FDA and EMA. Key regulations include:
- FDA's 21 CFR Part 11, which outlines the criteria for electronic records and signatures.
- EU Annex 11, which regulates computerized processes in GMP-controlled environments.
These regulations mandate that all computerized networks utilized in the pharmaceutical sector must comply with to ensure they function accurately and generate dependable data. Compliance with these regulations not only mitigates risks associated with data integrity but also enhances the overall quality of pharmaceutical products. Furthermore, companies must maintain comprehensive documentation to demonstrate compliance and facilitate audits by regulatory bodies.
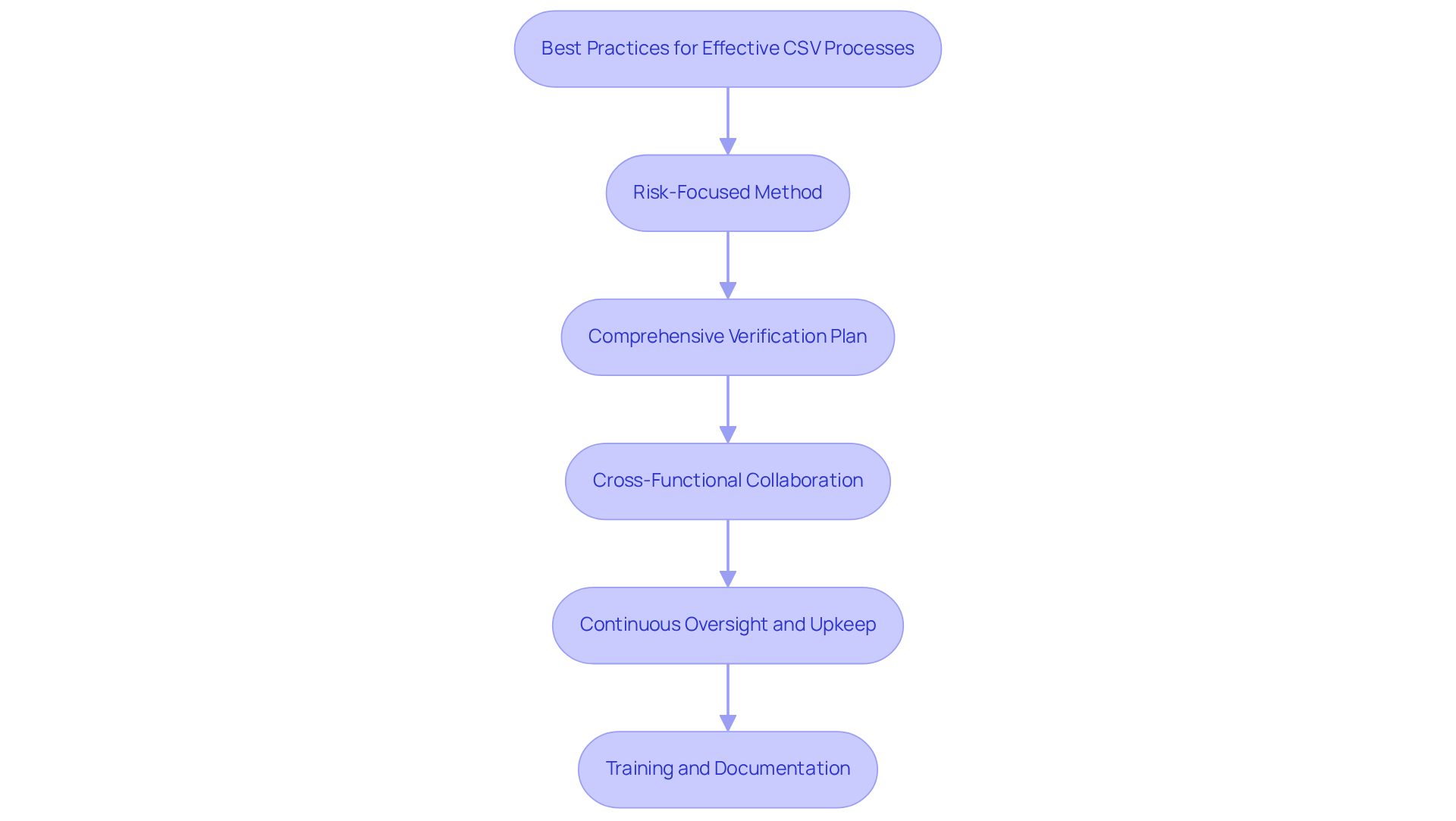
Implement Effective CSV Processes and Best Practices
To implement effective Computer System Validation (CSV) processes, organizations must adhere to several best practices that address compliance challenges and promote operational excellence:
- Risk-Focused Method: Prioritize verification efforts based on the associated risks of each framework. Allocate resources strategically to systems that significantly impact product quality and patient safety. This targeted approach not only enhances compliance but also improves operational efficiency.
- Comprehensive Verification Plan: Develop a detailed verification plan outlining the scope, objectives, and methodologies for verification activities. This plan should encompass testing protocols, documentation requirements, and timelines, ensuring a structured approach to verification that leaves no room for ambiguity.
- Cross-Functional Collaboration: Foster cooperation among IT, quality assurance, and operational teams to incorporate diverse perspectives during the validation phase. Regular meetings and clearly defined roles enhance communication and streamline efforts, ultimately leading to more effective outcomes.
- Continuous Oversight and Upkeep: Implement procedures for ongoing monitoring of validated processes to ensure sustained compliance. This includes regular evaluations, change control procedures, and re-validation when significant modifications occur, safeguarding the integrity of systems over time.
- Training and Documentation: Provide involved in CSV activities while maintaining precise records of all validation efforts. This documentation serves as critical evidence of compliance and is essential during regulatory audits, reinforcing the organization’s commitment to quality.
By adopting these best practices, pharmaceutical companies can fortify their initiatives in computer system validation in pharma, mitigate compliance risks, and uphold the integrity of their data and processes.
Conclusion
Computer system validation (CSV) in the pharmaceutical industry is an essential process that guarantees technology consistently meets defined specifications and quality standards. This systematic approach not only safeguards data integrity but also plays a pivotal role in maintaining compliance with regulatory requirements and enhancing patient safety. By implementing robust validation practices, pharmaceutical companies can achieve greater operational efficiency and reliability, ultimately contributing to the overall success of their compliance efforts.
Key arguments emphasize the necessity of adhering to regulatory requirements established by agencies such as the FDA and EMA, including critical guidelines like FDA's 21 CFR Part 11 and EU Annex 11. These regulations mandate that computerized systems in the pharmaceutical sector must be validated to ensure accurate functioning and dependable data generation.
Furthermore, effective CSV processes and best practices are outlined, such as:
- Adopting a risk-focused approach
- Fostering cross-functional collaboration
- Maintaining comprehensive documentation
All of which are crucial for mitigating compliance risks.
Given the increasing complexity of pharmaceutical operations and stringent regulatory landscapes, the significance of computer system validation cannot be overstated. Organizations must prioritize CSV not merely as a compliance requirement but as a fundamental aspect of their quality assurance processes. By committing to effective CSV practices, pharmaceutical companies can enhance product quality, ensure patient safety, and build trust with regulatory bodies and consumers alike. Embracing this proactive approach will not only facilitate compliance success but also position companies for sustainable growth in an ever-evolving industry landscape.
Frequently Asked Questions
What is computer system validation (CSV) in the pharmaceutical industry?
Computer system validation in pharma is a systematic and documented process that ensures computer technologies consistently deliver results that meet defined specifications and quality standards, safeguarding data integrity and ensuring patient safety.
Why is computer system validation important in pharma?
CSV is important for safeguarding data integrity, complying with regulatory requirements, and ensuring patient safety. It helps maintain the integrity of electronic records and positively impacts overall product quality.
What activities are involved in computer system validation?
CSV involves a comprehensive series of activities, including architecture design, rigorous testing, and ongoing monitoring to confirm that systems function as intended throughout their lifecycle.
How does effective validation practices benefit pharmaceutical companies?
By adopting effective validation practices, pharmaceutical companies can significantly improve operational efficiency, reliability, and adherence to Good Manufacturing Practices (GMP) and other regulatory frameworks.
