Master CFR 820 Compliance: Key Steps for Pharmaceutical Officers

Overview
The article delineates critical steps for pharmaceutical officers to achieve mastery in CFR 820 compliance, underscoring the significance of grasping the Quality System Regulation (QSR) for proficient quality management in medical device manufacturing. It substantiates this by detailing vital requirements, including:
- Design controls
- Documentation practices
- Corrective actions
Additionally, it accentuates the necessity for thorough training and proactive strategies to alleviate prevalent compliance challenges.
Introduction
Navigating the complexities of CFR 820 compliance is paramount for pharmaceutical officers tasked with ensuring that medical devices meet stringent quality standards. This regulation, known as the Quality System Regulation (QSR), outlines essential requirements that directly impact product safety and efficacy.
As the FDA intensifies scrutiny, the challenge lies in effectively implementing these compliance measures while fostering a culture of quality within organizations. How can pharmaceutical firms not only meet these regulatory demands but also leverage them to enhance their competitive edge in a rapidly evolving market?
By addressing these challenges head-on, organizations can transform compliance from a mere obligation into a strategic advantage, driving innovation and success in the industry.
Understand the Basics of CFR 820 Compliance
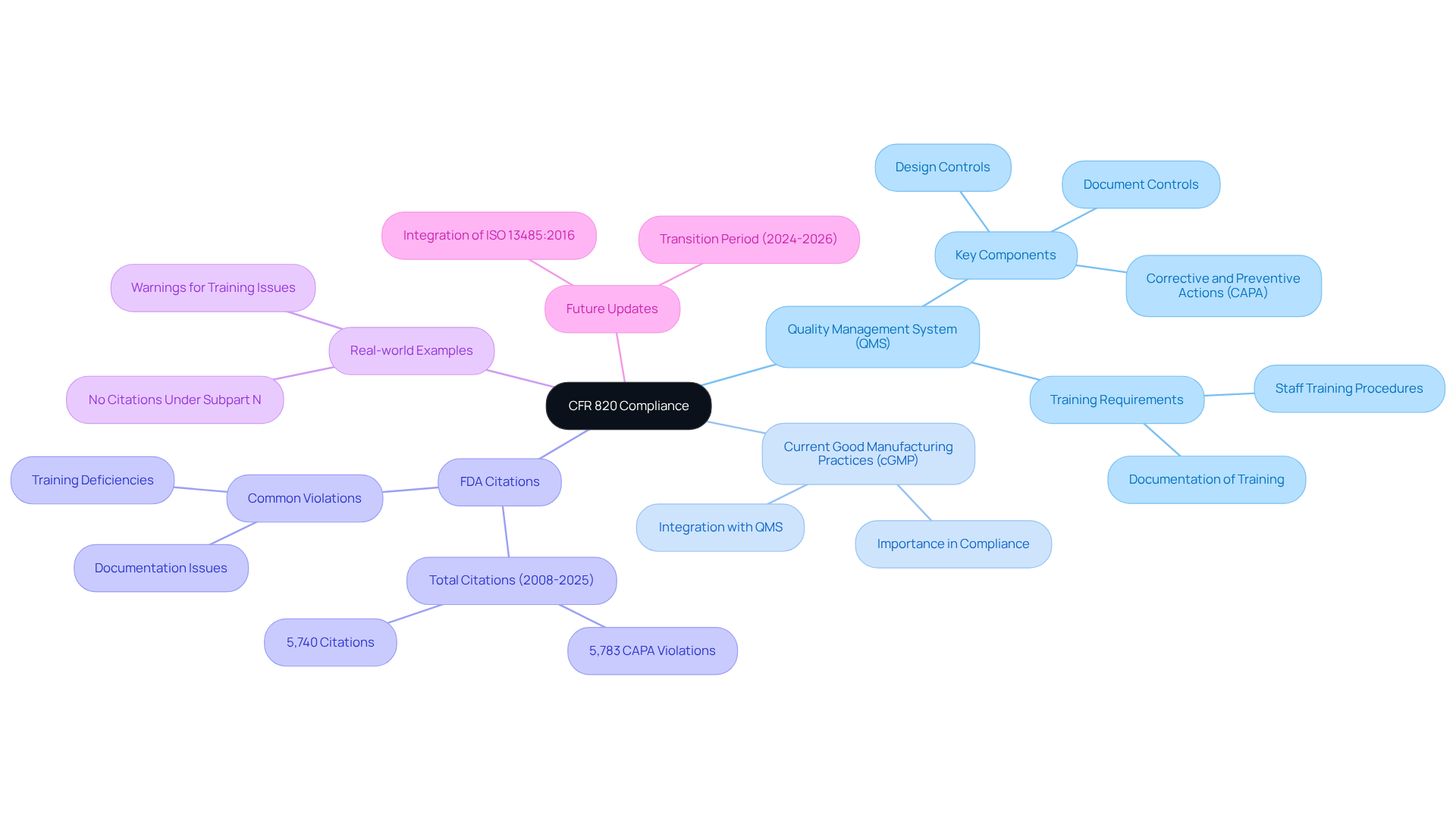
The regulations outlined in cfr 820, recognized as the Quality System Regulation (QSR), delineate critical FDA guidelines governing the quality management systems (QMS) of medical device manufacturers. This regulation stipulates requirements across various stages, including design, manufacturing, packaging, labeling, storage, installation, and servicing of medical devices. For pharmaceutical regulatory officers, comprehending these fundamentals is crucial for ensuring compliance with necessary standards. Key concepts such as current Good Manufacturing Practices (cGMP) and QMS are integral to a robust adherence strategy.
The importance of cGMP and QMS in compliance with cfr 820 cannot be overstated. A recent analysis revealed that from 2008 to 2025, FDA inspections resulted in 5,740 citations under cfr 820, underscoring the prevalence of regulatory challenges within the industry. Notably, deficiencies in Corrective and Preventive Action (CAPA) systems ranked among the most cited violations, with 5,783 citations issued during the same timeframe. This underscores the imperative for pharmaceutical firms to implement that not only fulfill compliance requirements but also foster continuous improvement.
Real-world examples further underscore the significance of a robust QMS. For instance, no FDA citations were recorded under Subpart N from 2008 to 2025, indicating that manufacturers consistently meet expectations regarding servicing controls. In contrast, the FDA issued 46 warnings to device manufacturers concerning staff training, with nearly five percent citing insufficient procedures for training and identifying training needs. These instances highlight the critical role of comprehensive training and documentation in ensuring compliance.
As of 2025, updates to the FDA Quality System Regulation will integrate ISO 13485:2016 as the core standard, emphasizing the necessity for organizations to adapt to evolving regulatory landscapes. Industry leaders stress the importance of understanding cfr 820, with one asserting that cultivating a quality-focused culture is essential for survival in a stringent regulatory environment. By proactively monitoring and addressing non-conformances, companies can mitigate regulatory risks and enhance their competitive position.
In conclusion, a comprehensive understanding of the Quality System Regulation is vital for pharmaceutical regulatory officers. By prioritizing cGMP and implementing effective QMS, organizations can adeptly navigate the complexities of regulatory compliance and ensure the safety and efficacy of their medical devices.
Identify Key Requirements of CFR 820
CFR 820 outlines several key requirements that manufacturers must adhere to, including:
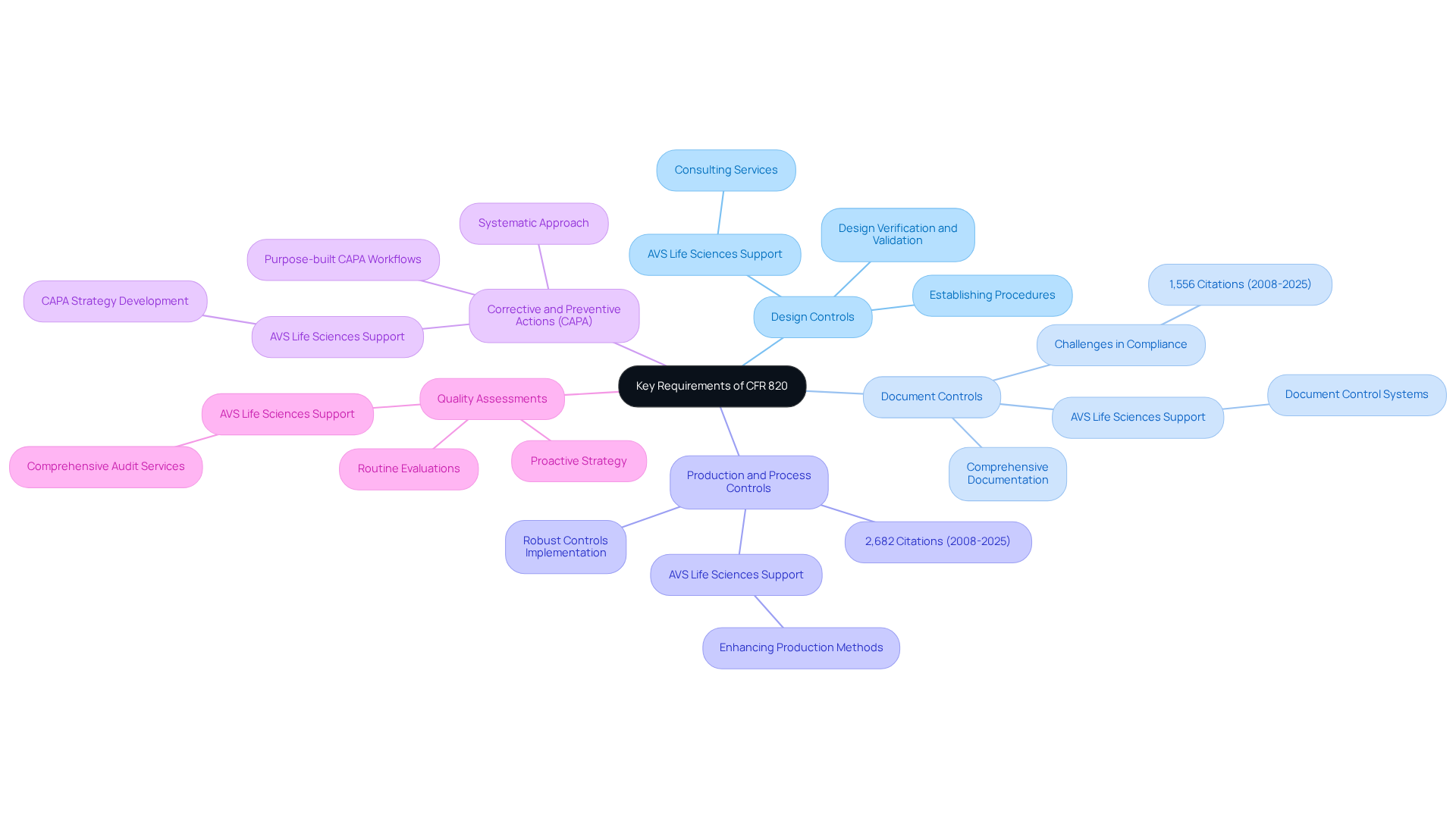
- Design Controls: Establishing procedures for design and development is crucial to ensure that devices meet user needs and intended uses. This involves rigorous design verification and validation processes to confirm that outputs align with inputs and fulfill intended purposes. AVS Life Sciences provides consulting services that assist organizations in establishing effective design controls, thereby ensuring adherence and enhancing product standards.
- Document Controls: It is essential to maintain comprehensive documentation that demonstrates compliance with the regulation, including design history files and device master records. Effective document control is vital, as evidenced by the 1,556 citations issued under Subpart D from 2008 to 2025, highlighting ongoing challenges in this area. AVS Life Sciences can assist in developing robust document control systems tailored to specific organizational needs.
- Production and Process Controls: Implementing robust controls is necessary to ensure that manufacturing processes are consistent and yield quality products. From 2008 to 2025, the FDA issued 2,682 citations under Subpart G, primarily due to poorly defined production procedures, underscoring the need for clear and effective controls. AVS Life Sciences offers expertise in enhancing production methods to comply with standards.
- Corrective and Preventive Actions (CAPA): Establishing a systematic approach for identifying and addressing nonconformities, as well as preventing their recurrence, is critical. Clear criteria for when CAPA is necessary are essential, along with the use of purpose-built CAPA management workflows to streamline processes. AVS Life Sciences can guide organizations in developing effective CAPA strategies that align with regulatory expectations.
- Quality Assessments: Performing routine evaluations to gauge adherence to the standards system and pinpoint areas for enhancement is a proactive strategy that assists organizations in staying ahead of possible regulatory challenges while promoting a culture of ongoing enhancement. AVS Life Sciences offers comprehensive audit services to ensure adherence to CFR 820 standards.
Statistics indicate that a significant percentage of manufacturers struggle with meeting the , with FDA inspectors issuing 5,740 citations under 21 CFR 820.30 from 2008 to 2025, primarily related to design validation. Successful instances of design controls in medical device firms demonstrate that following these requirements not only guarantees conformity but also enhances product quality and safety.
Recent updates from the FDA regarding design controls emphasize the importance of maintaining rigorous documentation and implementing effective design verification processes. Compliance officers have noted that a structured approach to design controls is vital for navigating the complexities of regulatory requirements and achieving successful outcomes in product development. Engaging with AVS Life Sciences can provide the necessary support and expertise to navigate these challenges effectively.
Implement Strategies for CFR 820 Compliance
To effectively implement CFR 820 compliance strategies, consider the following steps:
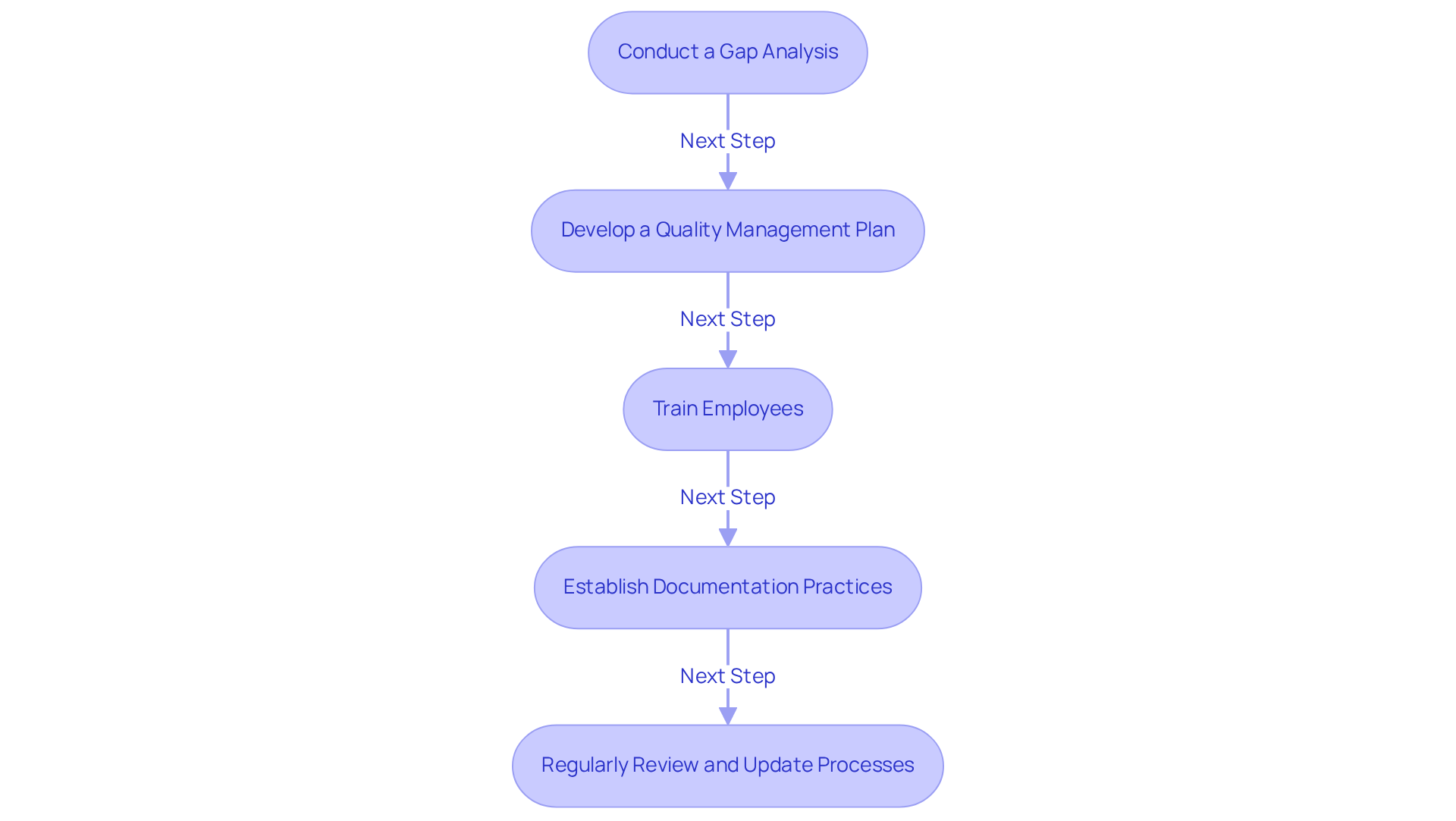
- Conduct a Gap Analysis: Begin by evaluating your current management system against CFR 820 requirements to identify areas needing enhancement. This analysis is crucial, as many organizations struggle with documenting closed-loop traceability and lack . Engaging with AVS Life Sciences can provide expert consulting to navigate these challenges effectively, including tailored assessments and recommendations.
- Develop a Quality Management Plan: Create a comprehensive plan that outlines how your organization will meet the CFR 820 requirements, including timelines and responsibilities. Statistics indicate that organizations with a strong management plan experience a significant reduction in compliance-related issues. AVS Life Sciences offers consulting services to assist you in creating and implementing this plan, ensuring conformity with compliance expectations.
- Train employees by offering training for all staff on the requirements of CFR 820 and the significance of adherence to cultivate a culture of quality. Effective training programs are crucial, as they equip staff with the necessary skills to ensure compliance with standards. AVS Life Sciences can assist in creating customized training modules tailored to your organization’s needs.
- Establish Documentation Practices: Implement robust documentation practices to ensure that all processes are recorded and easily accessible for audits. Maintaining thorough documentation is essential for adherence to CFR 820 during regulatory audits. AVS Life Sciences provides guidance on best practices for documentation to guarantee compliance and preparedness for inspections.
- Regularly Review and Update Processes: Continuously monitor and update your quality management system to adapt to changes in regulations and industry best practices. Organizations that consistently assess their procedures are better equipped to respond to changing regulatory requirements. Collaborating with AVS Life Sciences ensures that you remain informed of regulatory changes and uphold compliance through ongoing support and updates.
By implementing these strategies and leveraging the expertise of AVS Life Sciences, pharmaceutical officers can establish a proactive regulatory environment that mitigates risks and enhances product standards. For tailored consulting solutions in quality management and regulatory adherence, reach out to AVS Life Sciences today!
Troubleshoot Common CFR 820 Compliance Issues
Organizations striving for compliance with CFR 820 often encounter several common issues that can jeopardize their regulatory standing:
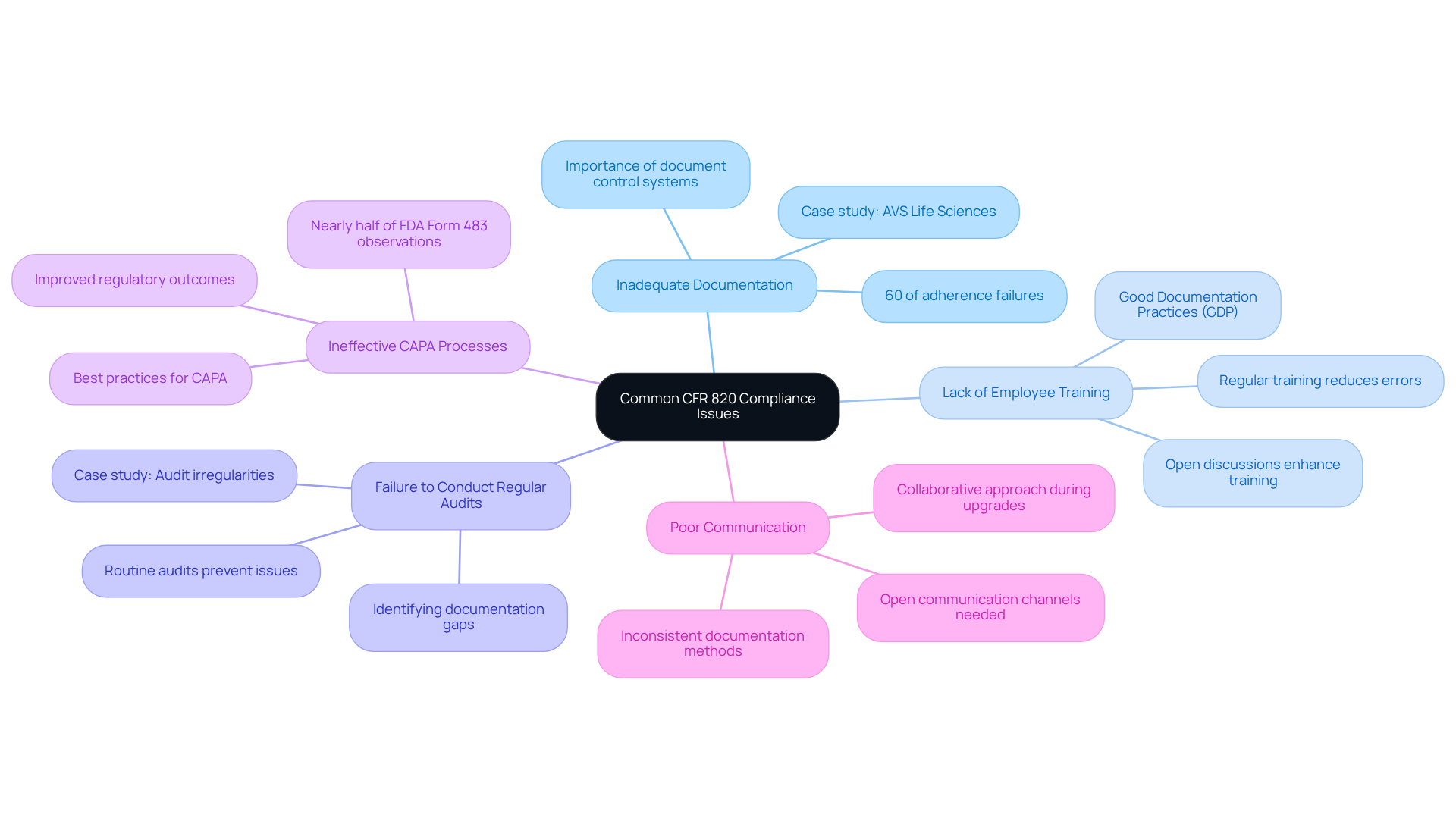
- Inadequate Documentation: Comprehensive documentation is essential for meeting requirements. Statistics indicate that 60% of adherence failures arise from insufficient or faulty documentation. Implementing a robust document control system in accordance with CFR 820 is crucial for managing revisions and approvals effectively. A recent case study by AVS Life Sciences with a leading biotechnology company underscores the importance of thorough documentation; our efforts to demonstrate full traceability were deemed appropriate by the client’s quality assurance team during their upgrade to a Level 2 GMP facility.
- Lack of Employee Training: Regular training on regulatory requirements is vital. Organizations that invest in training programs can significantly reduce documentation errors and improve overall adherence. Training should encompass (GDP) principles in accordance with CFR 820, ensuring that all personnel understand their roles in maintaining quality standards. Our collaboration with the client also highlighted the necessity for open discussions regarding team workload and responsibilities, which can enhance training effectiveness.
- Failure to Conduct Regular Audits: Internal audits are essential for identifying documentation gaps in accordance with CFR 820 before they develop into regulatory issues. Carrying out routine audits assists organizations in resolving documentation problems swiftly, averting possible regulatory failures. In our case study, we noted irregularities in test results that triggered a reassessment of the client’s audit procedures, resulting in valuable lessons learned and enhanced adherence outcomes.
- Ineffective CAPA Processes: A structured Corrective and Preventive Action (CAPA) process is essential. Best practices include thorough documentation and regular performance reviews, as recommended by CFR 820, to efficiently address quality issues. Notably, nearly half of all FDA Form 483 observations relate to CAPA failures, underscoring the need for effective implementation. Our experience with the client demonstrated how addressing gaps in their CAPA processes can lead to improved regulatory outcomes.
- Poor Communication: Open communication channels within the organization are necessary to address regulatory concerns promptly. Inconsistent documentation methods across departments can result in confusion and mistakes, complicating adherence. Establishing clear communication protocols can help mitigate these risks. The collaborative approach taken during the facility upgrade facilitated better communication and understanding of responsibilities among the teams involved.
By proactively addressing these issues, pharmaceutical officers can significantly enhance their organization's compliance stance, mitigate risks, and reduce the likelihood of penalties. As emphasized by industry experts, maintaining comprehensive records is not merely a regulatory requirement but a fundamental aspect of ensuring product safety and effectiveness.
Conclusion
Mastering CFR 820 compliance is not merely advisable; it is essential for pharmaceutical officers to ensure their organizations meet the stringent quality standards set forth by the FDA. By understanding the intricacies of the Quality System Regulation (QSR), professionals can navigate regulatory challenges effectively while fostering a culture of quality, which is crucial for the success of medical device manufacturing.
Key points throughout this article underscore the critical role of current Good Manufacturing Practices (cGMP) and effective Quality Management Systems (QMS). The necessity of robust documentation, comprehensive employee training, and systematic approaches to Corrective and Preventive Actions (CAPA) are highlighted as fundamental elements for achieving compliance. Real-world examples and statistical insights reinforce the importance of adhering to these regulations to avoid common pitfalls, such as inadequate documentation and ineffective training.
In today's rapidly evolving regulatory landscape, the significance of proactive compliance strategies cannot be overstated. Engaging with expert resources and consulting services empowers pharmaceutical organizations to not only meet but exceed compliance expectations. By prioritizing quality and embracing continuous improvement, companies can enhance operational efficiency, mitigate risks, and ultimately contribute to the safety and efficacy of medical devices in the marketplace. This commitment to excellence is not just a regulatory requirement; it is a strategic advantage that positions organizations for success.
Frequently Asked Questions
What is CFR 820 and what does it regulate?
CFR 820, known as the Quality System Regulation (QSR), outlines FDA guidelines governing the quality management systems (QMS) of medical device manufacturers. It specifies requirements for various stages including design, manufacturing, packaging, labeling, storage, installation, and servicing of medical devices.
Why is understanding CFR 820 important for pharmaceutical regulatory officers?
Understanding CFR 820 is crucial for pharmaceutical regulatory officers to ensure compliance with necessary standards and to effectively manage the quality of medical devices throughout their lifecycle.
What are current Good Manufacturing Practices (cGMP) and how do they relate to CFR 820?
Current Good Manufacturing Practices (cGMP) are essential practices that ensure products are consistently produced and controlled according to quality standards. They are integral to compliance with CFR 820 and contribute to a robust quality management strategy.
What were the findings regarding FDA inspections related to CFR 820?
From 2008 to 2025, there were 5,740 citations issued during FDA inspections under CFR 820, highlighting prevalent regulatory challenges. Notably, deficiencies in Corrective and Preventive Action (CAPA) systems were among the most cited violations, with 5,783 citations.
What real-world examples illustrate the importance of a robust QMS?
No FDA citations were recorded under Subpart N from 2008 to 2025, indicating effective servicing controls by manufacturers. Conversely, the FDA issued 46 warnings related to staff training, emphasizing the need for sufficient training procedures and documentation.
What changes are expected in the FDA Quality System Regulation by 2025?
By 2025, updates to the FDA Quality System Regulation will incorporate ISO 13485:2016 as the core standard, requiring organizations to adapt to the evolving regulatory landscape.
How can companies mitigate regulatory risks associated with CFR 820?
Companies can mitigate regulatory risks by proactively monitoring and addressing non-conformances and fostering a quality-focused culture, which is essential for survival in a stringent regulatory environment.
What is the overall conclusion regarding CFR 820 compliance for pharmaceutical firms?
A comprehensive understanding of the Quality System Regulation is vital for pharmaceutical regulatory officers. By prioritizing cGMP and implementing effective QMS, organizations can navigate regulatory complexities and ensure the safety and efficacy of their medical devices.
